EMBER-3 Trial KOL Discussion Leaders
EMBER-3 Key Trial Slides
EMBER-3 Trial Top Tweets
Top 10 posts ranked by impressions — click any card to view on X

A lot was covered but summary of 7 main studies during Metastatic HR+ #BreastCancer #SABCS highlights w/ @hoperugo: #AMBRE #MONALEESA #VIKTORIA1 #SERENA6 #evERA #EMBER3 #ASCENT07

The new #Halgorithm for treating HR+/HER2- metastatic breast cancer @DrHBurstein @DFCI_BreastOnc #SABCS24

Metastatic HR+ #BreastCancer #SABCS highlights w/ @hoperugo: #AMBRE #MONALEESA #VIKTORIA1 #SERENA6 #evERA #EMBER3 #ASCENT07

#SABCS2025 Honored to present this exciting data. Efficacy seen regardless of mESR1 or mPIK3CA. EVERA also shows benefit with giredestrant/EVE across mESR1 as did EMBER3. @OncoAlert

#ESMOBreast25 is in 5 days! EMBER-3 Subgroup Analysis: Imlunestrant + abema improved PFS after CDK4/6i. No abema benefit after abema. Consistent benefit in ESR1+ and PI3K-mutant.

#SABCS24 Part 2: Highlights w/ @jamecancerdoc — #EUROPA #TAILORx #PADMA #EMBER3

Key oral abstracts in breast cancer from #ASCO25 covering neoadjuvant, adjuvant & metastatic settings: INAVO120, EMBER-3, VERITAC-2, DESTINY-Breast06, AXSANA, I-SPY2 & more!

#SABCS24 beautiful discussion by @DrHBurstein on EMBER3. One of the best!! A fabulous and well qualified accumulation of data. @OncoAlert

Educational Session: After CDK4/6 Inhibitors — Advancing Treatment for HR+HER2-negative Metastatic Breast Cancer. Treatment strategies after CDK4/6 inhibitor progression. #SABCS25

Careful about drawing conclusions on abema after abema based on 23 patients. The HR confidence intervals are incredibly wide (0.37–2.31)
About the EMBER-3 Trial
The EMBER-3 trial is a Phase 3, randomized, open-label study evaluating imlunestrant — a next-generation oral selective estrogen receptor degrader (SERD) — as monotherapy and in combination with abemaciclib, versus standard-of-care endocrine therapy in patients with ER+/HER2− advanced breast cancer previously treated with endocrine therapy. The trial enrolled patients both with and without ESR1 mutations, with approximately 65% having received prior CDK4/6 inhibitor therapy. Results presented at SABCS 2024 demonstrated that imlunestrant monotherapy improved PFS in patients with ESR1-mutated disease, while the combination showed benefit across all-comers. Updated OS data at SABCS 2025 showed an 11.4-month trend favoring imlunestrant in ESR1m patients, not yet statistically significant.
Trial Methodology & Results
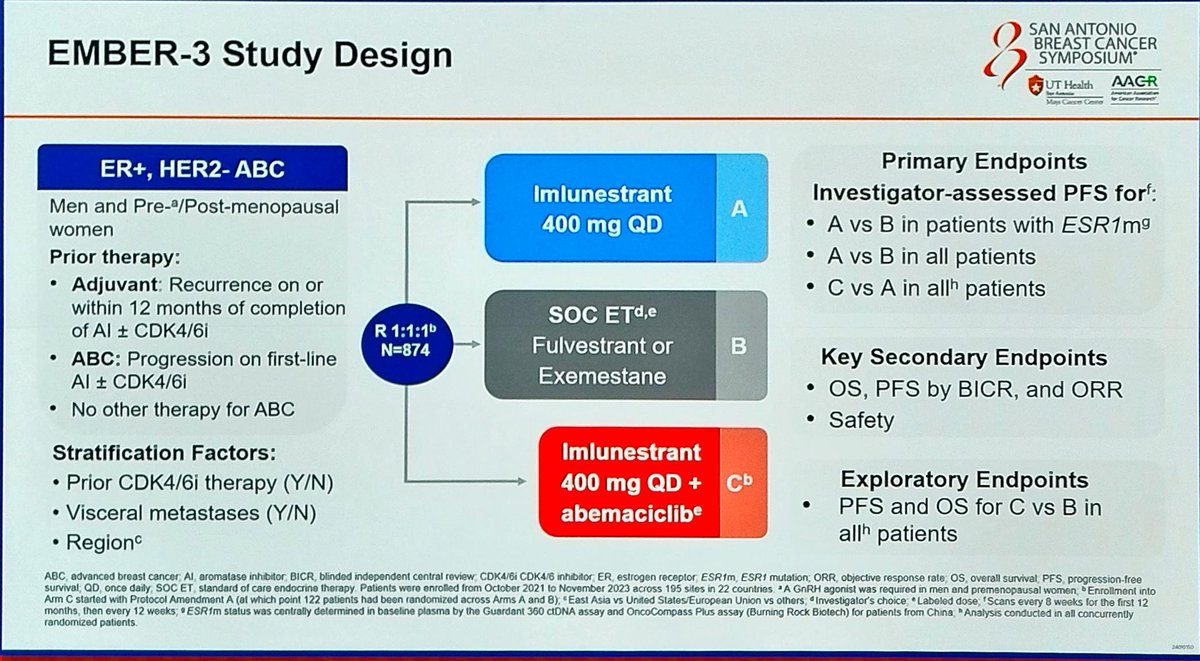
Study Design
Phase III, randomized, open-label, multi-center, active-controlled trial
Population
ER+/HER2− advanced breast cancer; ESR1-mutated and wild-type cohorts; ~65% prior CDK4/6i exposure
Interventions
(1) Imlunestrant monotherapy (2) Imlunestrant + Abemaciclib (3) SOC endocrine therapy (fulvestrant or AI)
Primary Endpoints
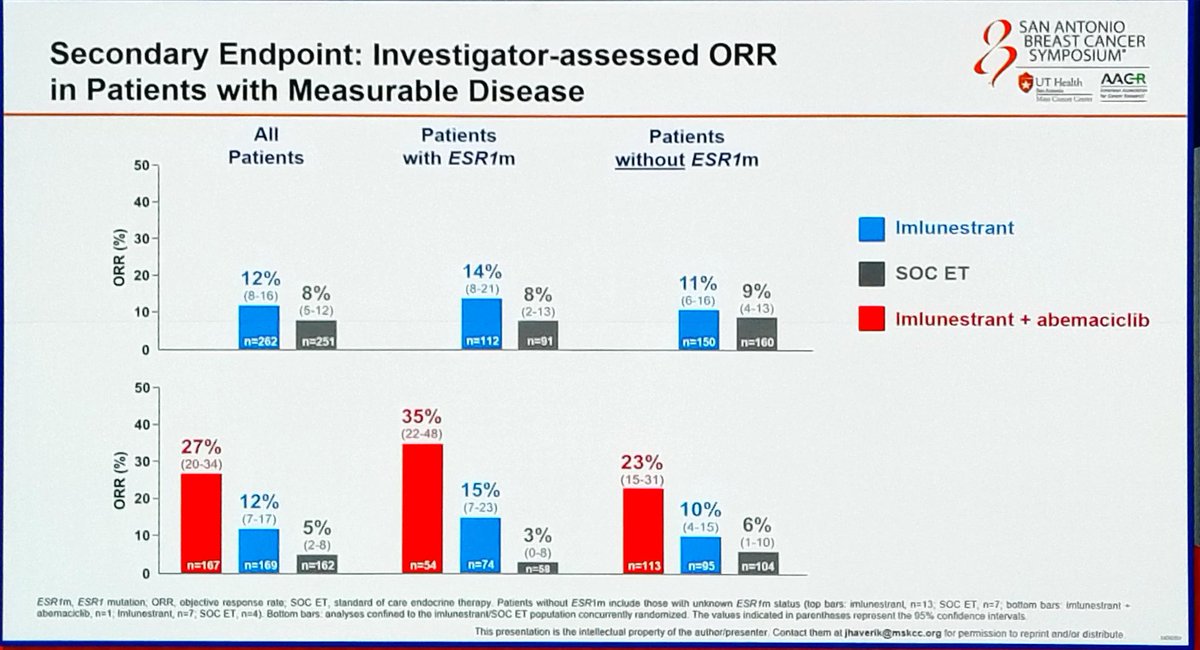
PFS in ESR1m population (imlunestrant vs. SOC ET) and all-comers (combo vs. imlunestrant alone). Secondary: OS, ORR, DoR, safety
Progression-Free Survival (PFS)
In the ESR1-mutated population, imlunestrant monotherapy demonstrated superior PFS vs. SOC ET (mPFS: 5.5 vs. 3.8 months; HR 0.62). Imlunestrant + abemaciclib significantly extended PFS across all patients (mPFS: 9.4 vs. 5.5 months; HR 0.57, p<0.0001). Updated data at ESMO Breast 2025 confirmed mPFS of 9.1 vs. 3.7 months in the post-CDK4/6i subgroup.
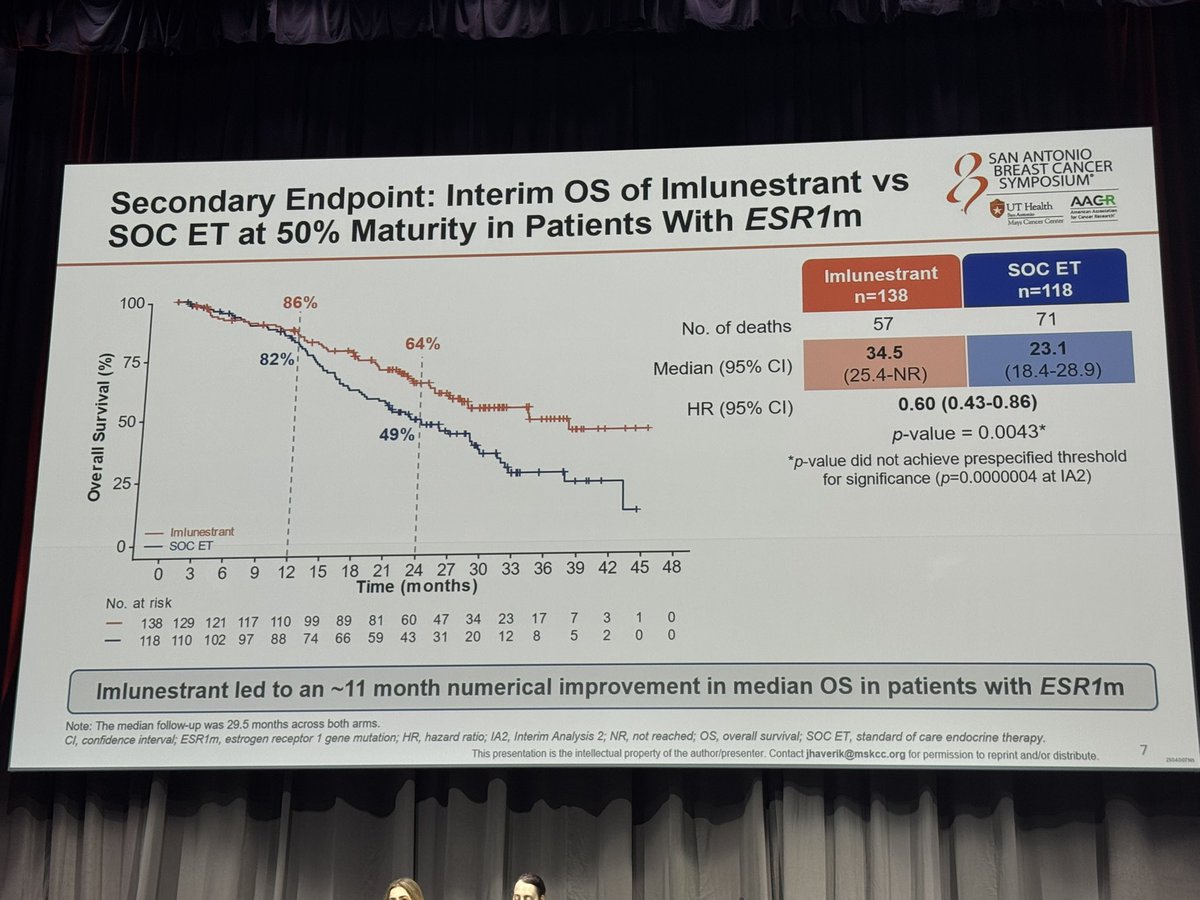
Overall Survival (OS)
At SABCS 2025, updated OS data showed an 11.4-month numerical improvement in the ESR1-mutated population for imlunestrant vs. SOC ET, though this did not yet reach statistical significance. FDA approved imlunestrant in September 2025 for HR+/mESR1 advanced breast cancer based on the PFS benefit. OS follow-up continues.
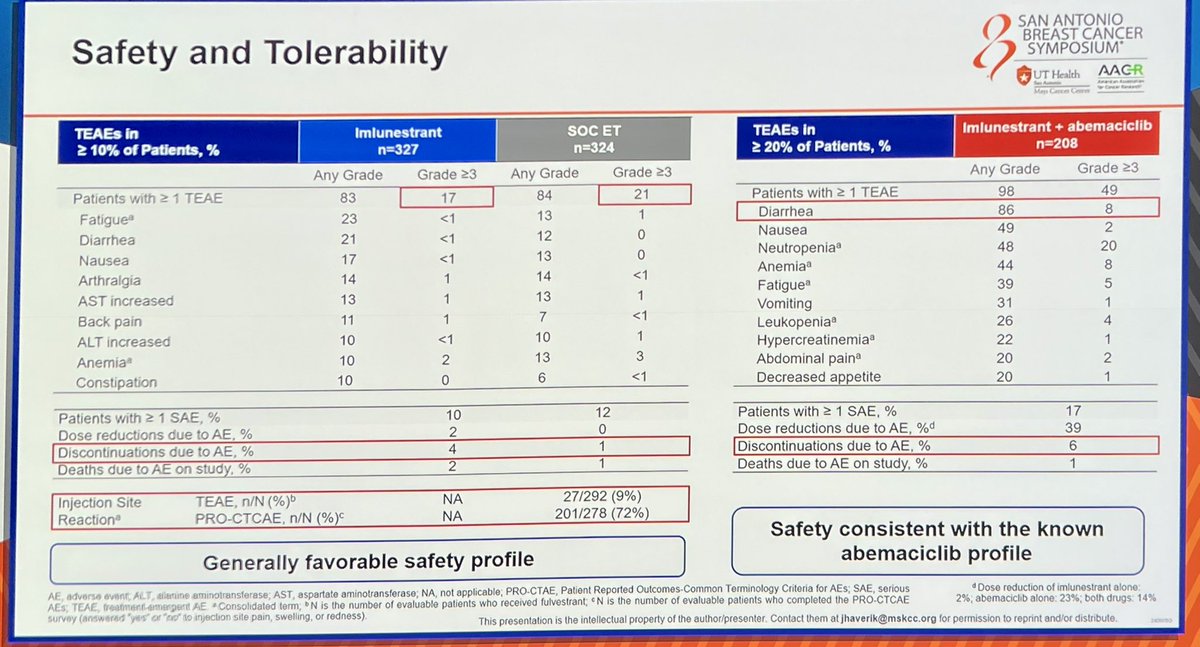
Safety & Tolerability
Imlunestrant demonstrated a favorable safety profile as monotherapy with minimal GI toxicity and no class-specific concerns. The combination with abemaciclib showed expected hematologic and GI adverse events consistent with CDK4/6i class effects; approximately 9% of combination patients experienced Grade ≥3 adverse events.
Clinical Implications
EMBER-3 positions imlunestrant as the second oral SERD (alongside elacestrant) approved for ESR1-mutated HR+ advanced breast cancer. The combination with abemaciclib represents a first-in-class all-oral CDK4/6i + SERD regimen showing benefit even after prior CDK4/6i exposure. Key open questions include the role of ctDNA clearance to guide de-escalation and optimal sequencing relative to chemotherapy and other SERDs.