Breast Cancer
EMBER3
About the EMBER3 Trial
The EMBER-3 trial is a randomized study evaluating the efficacy of imlunestrant, an oral selective estrogen receptor degrader (SERD), in patients with estrogen receptor-positive (ER+), HER2-negative advanced breast cancer. The study compares imlunestrant, both as monotherapy and in combination with abemaciclib, against standard-of-care (SOC) endocrine therapy. Primary endpoints include investigator-assessed progression-free survival (PFS) in patients with ESR1 mutations for imlunestrant versus SOC, and in the overall population for imlunestrant plus abemaciclib versus imlunestrant alone. Key secondary endpoints include overall survival (OS), PFS assessed by blinded independent central review (BICR), and objective response rate (ORR).
The results demonstrate that imlunestrant monotherapy significantly improves PFS in ESR1-mutant patients (HR = 0.62; 95% CI, 0.46–0.82). Furthermore, the combination of imlunestrant and abemaciclib yields a notable PFS benefit across all patients (HR = 0.57; 95% CI, 0.44–0.73), with a median PFS of 9.4 months compared to 5.5 months with imlunestrant alone. These findings were presented at the 2024 San Antonio Breast Cancer Symposium (SABCS), where key opinion leaders including Dr. Gaia Griguolo, Dr. Stephanie Graff, Dr. Francesco Schettini, and the Oncology Brothers engaged in in-depth discussions of the data.
Trial Methodology
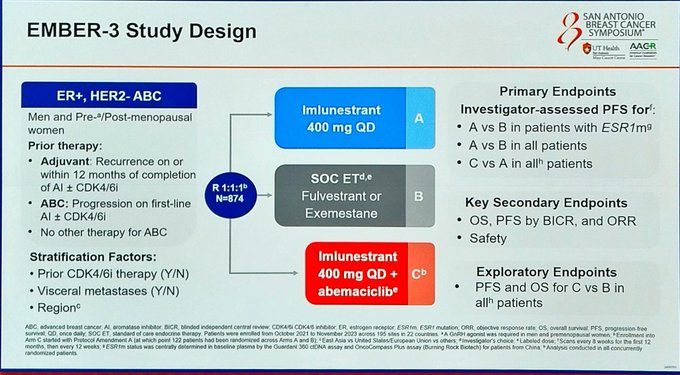
Study Design
Phase III, randomized, open-label, multi-center, active-controlled trial (EMBER-3)
Population
ER+/HER2− advanced breast cancer patients previously treated with endocrine therapy; ESR1-mutated and ESR1 wild-type cohorts; ~65% with prior CDK4/6 inhibitor exposure
Interventions
Three arms: (1) Imlunestrant monotherapy, (2) Imlunestrant + Abemaciclib combination, (3) Standard-of-care endocrine therapy (fulvestrant or aromatase inhibitor)
Primary Endpoints
Progression-free survival (PFS) in ESR1-mutated population (imlunestrant vs. SOC ET) and in all-comers (combination vs. imlunestrant alone). Secondary: OS, ORR, DoR, safety
Progression-Free Survival (PFS)
In the ESR1-mutated population, imlunestrant monotherapy demonstrated superior PFS compared to standard-of-care endocrine therapy (mPFS: 5.5 vs. 3.8 months; HR 0.62), while imlunestrant + abemaciclib significantly extended PFS across all patients regardless of ESR1 status (mPFS: 9.4 vs. 5.5 months; HR 0.57, p<0.0001). Updated data at ESMO Breast 2025 confirmed mPFS of 9.1 vs. 3.7 months in the post-CDK4/6i subgroup.
Overall Survival (OS)
At SABCS 2025, updated OS data showed an 11.4-month improvement in the ESR1-mutated population for imlunestrant monotherapy vs. SOC ET, though this did not yet reach statistical significance. The FDA approved imlunestrant in September 2025 for HR+/mESR1 advanced breast cancer based on the PFS benefit. OS follow-up continues.
Safety & Tolerability
Imlunestrant demonstrated a favorable safety profile as monotherapy — minimal GI toxicity with no class-specific concerns. The combination with abemaciclib showed expected hematologic and GI adverse events consistent with CDK4/6 inhibitor class effects; approximately 9% of combination patients experienced Grade ≥3 adverse events. Discontinuation rates remained low.
Clinical Implications
EMBER-3 positions imlunestrant as the second oral SERD (alongside elacestrant) approved for ESR1-mutated HR+ advanced breast cancer. The combination with abemaciclib represents a first-in-class all-oral CDK4/6i + SERD regimen showing benefit even after prior CDK4/6i exposure. Key open questions include the role of molecular response (ctDNA clearance) to guide de-escalation, and the optimal sequencing relative to chemotherapy and other SERDs. Shared decision-making around tolerability and OS data maturity remains central to clinical adoption.
