Lung Cancer
AEGEAN
About the AEGEAN Trial
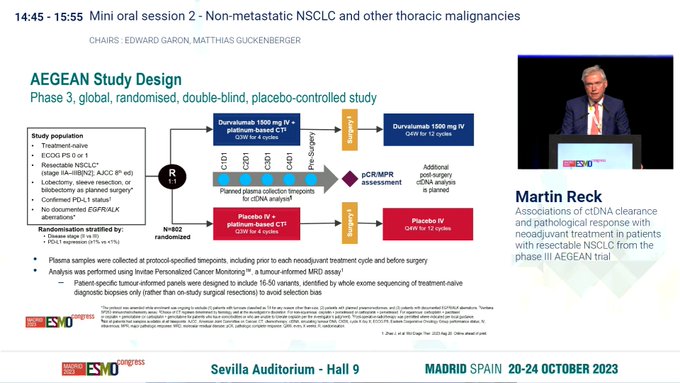
The AEGEAN trial is a Phase III, randomized, double-blind study evaluating the efficacy of perioperative durvalumab in patients with resectable stage IIA to IIIB (N2) non-small cell lung cancer (NSCLC). Participants received neoadjuvant durvalumab plus platinum-based chemotherapy followed by surgery, and then adjuvant durvalumab, compared to a control arm receiving placebo plus chemotherapy. The primary endpoint was event-free survival (EFS), with secondary endpoints including pathologic complete response (pCR) and major pathologic response (MPR). Durvalumab significantly improved EFS and pathologic response rates compared to placebo. Additional analyses showed higher rates of circulating tumor DNA (ctDNA) clearance and deeper pathological responses in the durvalumab arm. These results were presented by Dr. Martin Reck at the 2023 ESMO Congress in Madrid. See Dr. Hidehito Horinouchi for more online commentary.Table of Contents
Major Presentations and Milestones
AEGEAN Trial design, results, and conclusions
AEGEAN Sentiments and Criticisms
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
AEGEAN Trial: Major Presentations and Milestones
Primary speakers driving the story
Dr. John Heymach presented the results of AEGEAN at AACR23 with a discussion by Dr. Roy Herbst
Two giants in who are cracking the back of lung cancer! Heymach and Herbst @MDAndersonNews @YaleCancer Many lives impacted by their good work. 🙏👏#aacr23 pic.twitter.com/Wkeq7QuZC9
— Dr. Ron DePinho (@RonDePinho) April 16, 2023
Impressive data from #AEGEAN at #AACR23 from Dr. John Heymach and colleagues. This is the first of several phase III peri-operative IO studies in resectable NSCLC combining neoadjuvant chemo-immunotherapy followed by adjuvant immunotherapy. pic.twitter.com/uooR2C7GIG
— Stephen V Liu, MD (@StephenVLiu) April 16, 2023
Dr. Drew Moghanaki had an interesting perspective on the AEGEAN presentation by Dr. Heymach
The moment Heymach started clicking through his slides. #AACR23 pic.twitter.com/TpitW5EWy5
— Drew Moghanaki (@DrewMoghanaki) April 16, 2023
Dr. Mark Awad presentation at ASCO23 of KEYNOTE 671, discussing AEGEAN in the context of overlapping trials
Great discussion of KEYNOTE 671 by Dr. @DrMarkAwad at #ASCO23. Notes challenges of comparing studies given differences in populations. But EFS curves do appear to widen over time and plateau - perhaps more than CM816. And data more reassuring with longer f/u than AEGEAN. #LCSM pic.twitter.com/KihlunxaXo
— Stephen V Liu, MD (@StephenVLiu) June 3, 2023
Martin Reck, MD, PhD (ESMO23 ; ctDNA analysis highlighted by KOLs): “ctDNA potentially can be a predictive marker, often early.”
#ESMO23 @myESMO ctDNA analysis from AEGEAN - ctDNA potentially can be a predictive marker, often early @MartinReck2 #NSCLC pic.twitter.com/8jbuV8lLm3
— Charu Aggarwal, MD, MPH, FASCO (@CharuAggarwalMD) October 23, 2023
Hidehito Horinouchi, MD, PhD (ESMO 2023 session highlights): “AEGEAN: Association of ctDNA clearance and pathologic response … Earlier ctDNA CL was associated with higher likelihood of pCR and MPR.” https://twitter.com/HHorinouchi/status/1716442628106158577
NEJM (journal publication milestone): “Full AEGEAN trial Research Summary.” https://twitter.com/NEJM/status/1722267741644591118
Rami Manochakian, MD (ESMO 2023 + NEJM publication signal boost): “Just published @NEJM in conjunction with presentation @myESMO #ESMO23. ‘Results of #AEGEAN trial of #Perioperative #Durvalumab …’” https://twitter.com/RManochakian/status/1716443585933975700
2024, Oncology Brothers Discussion with Dr. Sandip Patel and Dr. Mara Antonoff regarding FDA Approval based on AEGEAN clinical trial
#LungCancer: we🗣️the @US_FDA approval of #Durvalumab (+ options in periOp/PostOp) for resectable NSCLC w/ @maraantonoff & @PatelOncology
— Oncology Brothers (@OncBrothers) October 20, 2024
Full Discussion:
- https://t.co/p16bUJj3Us
- https://t.co/wDr1Joi03B
- Also on "Oncology Brothers" podcast #OncTwitter #lcsm @TargetedOnc pic.twitter.com/QJCESM7wGS
KOL reactions to the presentation and its significance
-
Paolo Tarantino, MD: “2‑year EFS … 63.3% with neoadjuvant + adjuvant durva (AEGEAN, 16 cycles of IO).” https://twitter.com/PTarantinoMD/status/1689391490311524352
-
Nathan A. Pennell, MD, PhD, FASCO: “Based on the CM 816, Aegean and now KN 671, for stage 2/3 resectable NSCLC with no genomic alterations, do you give:” https://twitter.com/n8pennell/status/1665088564290527234
-
Tony Calles, MD: “ctDNA clearance and pathological response with neoadjuvant treatment in patients with resectable NSCLC from the phase III AEGEAN trial … Another @NEJM” https://twitter.com/Tony_Calles/status/1716441726401843469
-
Julien Mazieres, MD, PhD: “Interesting approach using ctDNA clearance as a surrogate marker of efficacy of neoadjuvant durvalumab + chemo (AEGEAN …)” https://twitter.com/JulienMazieres/status/1716442144859152718
-
Dipesh Uprety, MD: “AEGEAN … perioperative Durvalumab … → ↑ DFS with Durva” https://twitter.com/DipeshUpretyMD/status/1735164959640731710
AEGEAN Trial design, results, and conclusions
Core design/results (as amplified by KOLs)
AEGEAN: a phase III trial w/ pts w/ resectable stage IIA-IIIB NSCLC without EGFR/ALK-->randomized to neoadjuvant chemo (X4 cycles )± durva followed by adj placebo vs durva for 1 yr--> ↑ EFS with Durva #AACR23 @OncoAlert #LCSM pic.twitter.com/Nyf8fDqQVu
— Dipesh Uprety MD FACP (@DipeshUpretyMD) April 16, 2023
AEGEAN impressive pCR with durvalumab 4 cycles + chemotherapy and promising EFS. Contribution of adjuvant durvalumab remains unclear (vs CM816) : waiting more mature results with OS … ? #AACR23 pic.twitter.com/1VwxvkUY7Y
— d.planchard (@dplanchard) April 16, 2023
Overview amplified by KOLs
-
Setting: Resectable stage II–IIIB(N2) NSCLC; perioperative strategy with neoadjuvant durvalumab + platinum‑chemo, surgery, then adjuvant durvalumab vs matched placebo regimen. Primary endpoint: EFS; pCR/MPR key pathological endpoints.
- Source: session/thread highlights and slide captures
- https://twitter.com/HHorinouchi/status/1716442628106158577
- https://twitter.com/NEJM/status/1722267741644591118
-
Efficacy framing from KOLs: At 2 years, EFS around 63.3% in AEGEAN (neoadjuvant + adjuvant durvalumab) in cross‑trial context.
-
Publication and dissemination milestones: NEJM research summary and ESMO 2023 presentation alignment.
KOL deep‑dives on design/results
-
Hidehito Horinouchi, MD, PhD — Design and biomarker linkage: “Earlier ctDNA CL was associated with higher likelihood of pCR and MPR.” Emphasizes Phase III design, EFS primary endpoint, and NCT03800134 context. https://twitter.com/HHorinouchi/status/1716442628106158577
-
Charu Aggarwal, MD, MPH — Translational framing: “ctDNA potentially can be a predictive marker, often early.” https://twitter.com/CharuAggarwalMD/status/1716442186701508961
-
Paolo Tarantino, MD — Cross‑trial clinical anchoring: 2‑year EFS comparison across CM816, KN671, and AEGEAN, placing AEGEAN’s perioperative durvalumab in a competitive context. https://twitter.com/PTarantinoMD/status/1689391490311524352
Treatment landscape context
- NEJM — Curative‑intent surgery remains the cornerstone in early‑stage NSCLC, yet recurrence risk is substantial; AEGEAN situates perioperative IO to reduce events. https://twitter.com/NEJM/status/1722267741644591118
Subsets and deeper reads
Biomarker/ctDNA threads and mini‑oral details
-
ctDNA clearance association with pCR/MPR and early‑response utility:
-
Educational/media amplifiers:
- IASLC podcast with Narjust Florez, John Heymach, Teresa Reis: https://twitter.com/IASLC/status/1724449611363078215
- NEJM Research Summary: https://twitter.com/NEJM/status/1722267741644591118
Translational/MRD takeaways
🔥AEGEAN: Association of ctDNA clearance and pathologic response
— Hidehito HORINOUCHI (@HHorinouchi) October 23, 2023
🎙️@MartinReck2
🎯Earlier ctDNA CL was associated with higher likelihood of pCR and MPR.
✅Phase III
✅Primary: EFS
✅Stage II–IIIB(N2) NSCLC
✅NCT03800134#ESMO23 #LCSM @myESMO @oncoalert pic.twitter.com/LQTJE1sz1n
-
Tumor‑informed ctDNA (MRD) may function as an early‑response biomarker in AEGEAN:
- “ctDNA potentially can be a predictive marker, often early.” — Charu Aggarwal, MD https://twitter.com/CharuAggarwalMD/status/1716442186701508961
- “Earlier ctDNA CL was associated with higher likelihood of pCR and MPR.” — H. Horinouchi, MD, PhD https://twitter.com/HHorinouchi/status/1716442628106158577
PRO/QoL and duration pragmatics (KOL questions in practice)
-
Regimen selection and real‑world algorithms:
- “Based on the CM 816, Aegean and now KN 671 … do you give:” (adoption poll) — Nathan A. Pennell, MD, PhD https://twitter.com/n8pennell/status/1665088564290527234
-
Comparative maturity of follow‑up and cross‑trial interpretation:
- “EFS curves … appear to widen over time and plateau … And data more reassuring with longer f/u than AEGEAN.” — Stephen V. Liu, MD https://twitter.com/StephenVLiu/status/1664989062954987521
AEGEAN Trial Sentiments from KOLs: standard of care and critical appraisals
Strongly supportive/SoC‑leaning voices
Rami Manochakian, MD (NEJM + ESMO23 alignment; dissemination): https://twitter.com/RManochakian/status/1716443585933975700
Charu Aggarwal, MD, MPH (ctDNA predictive potential; translational enthusiasm): https://twitter.com/CharuAggarwalMD/status/1716442186701508961
Hidehito Horinouchi, MD, PhD (design clarity and ctDNA‑pCR/MPR linkage): https://twitter.com/HHorinouchi/status/1716442628106158577
Critical/contrarian perspectives (design, control arm, real‑world ethics, prevention)
Dr. Ido Wolf: Time Toxicity, Financial Toxicity: https://x.com/IdoWolf5/status/1647910420517122051
Stephen V. Liu, MD (cross‑trial maturity critique): “Notes challenges of comparing studies … EFS curves … widen … plateau … data more reassuring (with KEYNOTE 671) with longer f/u than AEGEAN.” https://twitter.com/StephenVLiu/status/1664989062954987521
Nathan Pennell, MD (strategy ambiguity): “Final results of neoadjuvant ChemoIO vs Neo+adj IO: clear as mud!”
Final results of neoadjuvant ChemoIO vs Neo+adj IO: clear as mud! https://t.co/hR7QuEUMud
— Nathan A. Pennell MD, PhD, FASCO (@n8pennell) June 4, 2023
Dr. Jack West: Cost Concern relative the Checkmate 816
Yes, new option, but is there anyone in whom you would say you (or anyone) should favor it over CM816? Particularly when you start treatment w/o knowing if pt will achieve pCR or not later?
— H. Jack West, MD, FASCO (@JackWestMD) April 17, 2023
We say "nice, another option" as if there's any value in having >1. It costs $200k more!
At the AACR23 discussion, Dr Roy Herbst stated that AEGEAN is a new standard of care but not yet the standard of care
@DrRoyHerbstYale giving a "Tour de Force" discussion of AEGEAN neoadjuvant-adjuvant trial in early stage NSCLC at AACR23. Concludes that this regimen is "a" new SOC but not yet "the" SOC, given other positive trials in this space. Awaiting ctDNA for MRD @AACR pic.twitter.com/sswpzVjcUR
— David Gandara (@drgandara) April 18, 2023
Dr. Naidoo shared the specific critique of AEGEAN by Dr. Herbst:
#AACR23 Is #AEGEAN a practice-changing study by @DrRoyHerbstYale?
— Jarushka Naidoo (@DrJNaidoo) April 16, 2023
- with similar outcomes to CM816, AEGEAN represents a new option of periop chemo-IO + 1 yr IO
- does not replace CM816
- does not address what adj IO adds, therefore a lateral move on practice@OncoAlert #LCSM pic.twitter.com/QtCYuGF21q
AEGEAN Temporal Sentiment Arc
AEGEAN Temporal Sentiment Arc (updated with AACR23)
2023 (AACR 2023 primary perioperative readout; AEGEAN enters the algorithm) Primary/KOL tweets:
#AACR23 Great presentation of #AEGEAN but more Qx arise-Some food for thought compared to #CM816:
— Giannis Mountzios (@g_mountzios) April 17, 2023
1/5
👉Histology and sex NOT a strata factor in AEGEAN
👉Pts had 4 cycles of neoadj chemo,might explain in part the slightly better perf. of PBO (25.9 m) compared to CM816 (20.8 m)? pic.twitter.com/vvx4nBfOP8
- https://x.com/StephenVLiu/status/1647688924037259264
- https://x.com/StephenVLiu/status/1647688926855847939
- https://x.com/StephenVLiu/status/1647688929347506177
- https://x.com/DrJNaidoo/status/1647684160381759488
- https://x.com/OncBrothers/status/1647638337849622528
- https://x.com/APassaroMD/status/1647676910279839744
- https://x.com/Tony_Calles/status/1647854634663399424
- Tone: Live AACR threads introduce AEGEAN’s perioperative durvalumab approach (neoadjuvant chemo‑IO → surgery → adjuvant IO) and early EFS/pathologic response signals. KOLs quickly elevate it alongside other perioperative programs and flag key questions around adjuvant duration, ctDNA/MRD, and patient selection. Shift: From anticipation to an “IO-perioperative era” frame, with immediate appetite for cross‑trial comparisons.
Mid‑2023 (ASCO 2023 discussant window; cross‑trial comparisons and pragmatics) Primary/KOL tweets:
Is it applicable for ANY patients?
— H. Jack West, MD, FASCO (@JackWestMD) May 17, 2023
But we will need to revise all of these thoughts in the wake of recent data presentations on AEGEAN, Neotorch, & upcoming #ASCO23 presentation of KEYNOTE-671. This field is evolving faster than we can commit the ideas to print. https://t.co/yLI2dycGkt
- https://twitter.com/StephenVLiu/status/1664989062954987521
- https://twitter.com/n8pennell/status/1665088564290527234
- https://twitter.com/JackWestMD/status/1658931655200088064
- https://twitter.com/DrewMoghanaki/status/1664091121968873472
- https://twitter.com/LeiDeng3/status/1665686515790368769
- Tone: KOLs compare perioperative (AEGEAN, KN‑671) versus neoadjuvant‑only (CM‑816) paradigms, cautioning about follow‑up maturity and pitfalls of cross‑trial reads. Polls and panel threads explore what clinicians should actually give on Monday, reflecting early real‑world decision tension. Shift: From AACR enthusiasm to measured, side‑by‑side appraisal and early adoption polling.
Late‑2023 (ESMO 2023 ctDNA analyses; NEJM publication; translational lift) Primary/KOL tweets:
🧬 cDNA clearance and pathological response with neoadjuvant treatment in patients with resectable NSCLC from the phase Ill AEGEAN trial@MartinReck2
— Dr. Antonio Calles 🫁🚭 (@Tony_Calles) October 23, 2023
Another @NEJM #ESMO23 #LCSM pic.twitter.com/mNCxlQleod
- https://twitter.com/CharuAggarwalMD/status/1716442186701508961
- https://twitter.com/HHorinouchi/status/1716442628106158577
- https://twitter.com/Tony_Calles/status/1716441726401843469
- https://twitter.com/JulienMazieres/status/1716442144859152718
- https://twitter.com/RManochakian/status/1716443585933975700
- https://twitter.com/NEJM/status/1722267741644591118
- https://twitter.com/JacobPlieth/status/1722544095283822687
- https://twitter.com/IASLC/status/1724449611363078215
- Tone: ctDNA clearance associations with pCR/MPR elevate MRD as a potential early‑response biomarker in perioperative NSCLC. NEJM publication plus ESMO visibility consolidate credibility and push biomarker‑informed conversations, with caveats about assay sensitivity and need for longer follow‑up. Shift: From regimen comparisons to biomarker‑enabled refinement and educational amplification.
2024 (education and comparative EFS framing) Primary/KOL tweets:
#WCLC24 Updated Analysis of AEGEAN perioperative durvalumab in early stage #NSCLC
— Giannis Mountzios (@g_mountzios) September 9, 2024
➡️PFS HR=0.69
➡️ OS=0.89, adjusted to 0.84 after censoring of COViD19 related deaths
➡️ Magnitude greater among PCR pts
➡️Improvemt in Lung-cancer related survival@IASLC #some #LCSM pic.twitter.com/TK27jmDWjB
- https://twitter.com/DipeshUpretyMD/status/1735164959640731710
- https://twitter.com/PTarantinoMD/status/1689391490311524352
- https://x.com/StephenVLiu/status/1797383735312044175
- https://x.com/g_mountzios/status/1833278304092299376
- https://x.com/OncBrothers/status/1848032082129236347
- Tone: As more centers design perioperative pathways, KOLs use summary visuals and cross‑trial EFS snapshots to anchor expectations. Practical debates continue on adjuvant duration, perioperative workflows, and where ctDNA could fit in decision-making. Shift: From data dissemination to operational playbooks and center‑specific implementation.
2025 (ongoing international reflections; optimization and adoption signals) Primary/KOL tweets:
Do we have any MRD (minimal residual doubt) that MRD assays will make a major impact in risk stratification in periop/peri-xrt patient management?
— Balazs Halmos (@BalazsHalmosMD) June 2, 2025
The plot thickens as to their clinical value/utility! https://t.co/hcB2RQ5c8G
- https://x.com/HHorinouchi/status/1925926945516048569
- https://x.com/HHorinouchi/status/1929295287501132202
- https://x.com/HHorinouchi/status/1930966680601088244
- https://x.com/BalazsHalmosMD/status/1929354704728826114
- https://x.com/CharuAggarwalMD/status/1928253525546652154
- https://x.com/jennifermarksmd/status/1929292670045741556
- https://x.com/MarceloCorassa/status/1929291300278309131
- Tone: Cross‑disciplinary and international posts highlight continued adoption with nuanced attention to assay sensitivity, sequencing, toxicity monitoring, and perioperative logistics. The conversation blends reaffirmation of perioperative IO value with methodologic caution and real‑world optimization.
The arc now clearly starts at AACR 2023 with AEGEAN’s perioperative durvalumab readout and evolves through ASCO 2023 comparisons, ESMO/NEJM translational lift, 2024 practice building, and 2025 refinement. Enthusiasm for perioperative IO is durable; debates have shifted toward how to integrate ctDNA, calibrate duration, and streamline perioperative workflows in routine care.
AEGEAN Professional Resources
