GI Cancer
BREAKWATER
About the BREAKWATER Trial
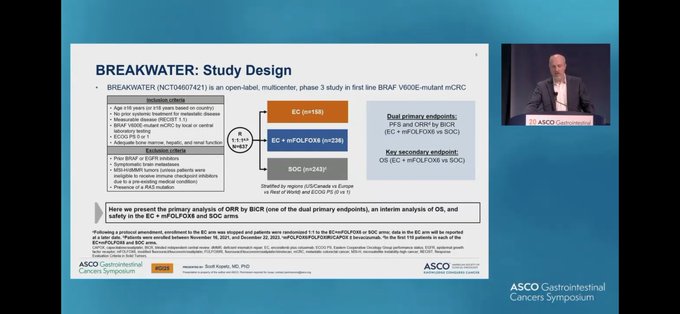
The BREAKWATER study is a randomized, multicenter Phase III trial evaluating the efficacy of targeted therapy in patients with previously untreated BRAF V600E-mutant metastatic colorectal cancer (mCRC). Patients were randomized 1:1:1 to receive encorafenib plus cetuximab with or without FOLFOX, or standard-of-care (SOC) chemotherapy with or without bevacizumab. The primary endpoint was objective response rate (ORR) as assessed by blinded independent central review (BICR), with secondary endpoints including progression-free survival (PFS), overall survival (OS), and safety.
The combination of encorafenib, cetuximab, and FOLFOX demonstrated a significantly higher ORR compared to SOC, with a trend toward improved OS in this treatment arm. The safety profile was consistent with known adverse event patterns associated with the individual agents, and toxicities were considered manageable. These results suggest enhanced clinical activity of targeted therapy when integrated into frontline treatment. The findings were presented at the 2024 ASCO Gastrointestinal Cancers Symposium and were discussed by leading experts, including Dr. Scott Kopetz and Krishan Jethwa, underscoring the evolving role of precision medicine in BRAF-mutant mCRC.
FDA approval noted in the tweet dataset: Oncology Brothers (context: BREAKWATER, BRAFV600E mCRC): “Chemo + EC is the SoC for BRAFV600E. This was @US_FDA approved in 2024!” https://x.com/OncBrothers/status/2010147873602633997
Table of Contents
Major Presentations and Milestones
BREAKWATER Trial design, results, and conclusions
BREAKWATER Sentiments and Criticisms
BREAKWATER Temporal Sentiment Arc
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
BREAKWATER Trial: Major Presentations and Milestones
Primary speakers driving the story
ASCO GI 2025 (#ASCOGI25): Early phase III BREAKWATER messaging emphasized that the trial met its primary endpoint for objective response rate (ORR) in treatment-naïve BRAF V600E–mutant metastatic colorectal cancer (mCRC). Cathy Eng, MD framed the ORR improvement and described an “early trend” toward OS, positioning the regimen as a new standard of care pending additional follow-up.
Results of Phase III BREAKWATER for BRAF V600E MT stage IV tx naive pts fulfilled primary endpoint for ORR (61% vs. 40%) and early trend for OS (add'l data to follow) defining a NEW standard of care. #colorectal #cancer @ASCO #ASCOGI25 #colorectal #CancerResearch @NatureMedicine… https://t.co/Wuk3JHDnbA https://t.co/2Ab6ki3ZAl
— Dr. Cathy Eng (@CathyEngMD) January 25, 2025
ASCO 2025 (#ASCO25): The conversation shifted from “ORR success” to mature time-to-event outcomes. Arndt Vogel, MD highlighted updated PFS and OS and explicitly called the regimen “new SOC in 1st line,” while also noting high grade ≥3 AE rates with the triplet approach.
1st line encorafenib + cetuximab + mFOLFOX6 vs SOC in BRAF V600E-mCRC
— Arndt Vogel (@ArndtVogel) May 30, 2025
🔎BREAKWATER: PFS + updated OS
#ASCO25
👉ORR 65 vs 37 vs 43 %
👉mPFS 12.8 vs 7.1 vs 6.9 mo
👉mOS 30.3 vs 15.1 vs 19.1 mo
👉AE ≥3 76% vs 58 vs 15.7%
🤔 Really effective, new SOC in 1st line
@myESMO https://t.co/bUqE8S053E
ESMO 2025 (#ESMO25): Attention broadened to translational correlates. Daisuke Kotani, MD, PhD amplified a ctDNA analysis suggesting consistent efficacy across baseline BRAF V600E VAF and highlighting differences in resistance mutation patterns by treatment exposure.
ASCO GI 2026 (#GI26): Follow-on analyses and cohort-level expansions were discussed, including a Cohort 3 readout using an irinotecan backbone (FOLFIRI) and post-hoc subgroup work (e.g., early-onset vs average-onset CRC), reflecting a shift from “does it work?” to “which backbone, which patients, and what biology?”
BREAKWATER Trial Design, Results, and Conclusions
Trial Design:
Based on the tweet dataset, BREAKWATER is a phase III program in previously untreated BRAF V600E–mutant mCRC evaluating targeted therapy integration with chemotherapy. The core comparison highlighted by multiple KOLs is:
Encorafenib + cetuximab + mFOLFOX6 versus standard-of-care chemotherapy (with additional comparator details referenced in some summaries).
A separate reported dataset at #GI26 describes BREAKWATER Cohort 3 evaluating encorafenib + cetuximab + FOLFIRI versus FOLFIRI ± bevacizumab.
Key Efficacy Results (as reported in tweets):
ASCO GI 2025 (primary endpoint focus): ORR improvement was emphasized.
- ORR: 61% vs 40% (Eng) https://x.com/CathyEngMD/status/1883270732987486704
ASCO 2025 (time-to-event outcomes): Updated PFS and OS were widely circulated.
- mOS: 30.3 vs 15.1 months; HR 0.49 (Eng) https://x.com/CathyEngMD/status/1928544745451635178
- mPFS: 12.8 vs 7.1 months (Vogel) https://x.com/ArndtVogel/status/1928541654832525604
GI26 (Cohort 3; irinotecan backbone):
- ORR: 64% vs 39% (p=0.001) (Martinez Lago) https://x.com/DraMartinezLago/status/2010104467920650638
- Early OS signal: “HR 0.49” (Martinez Lago) https://x.com/DraMartinezLago/status/2010104467920650638
Safety:
Safety was repeatedly framed as a key tradeoff with triplet therapy. Vogel reported:
- Grade ≥3 AEs: 76% vs 58% vs 15.7% (as presented in that tweet summary) https://x.com/ArndtVogel/status/1928541654832525604
Martinez Lago described Cohort 3 toxicity as “manageable” with “no new signals,” but no numeric AE rates were included in that tweet. https://x.com/DraMartinezLago/status/2010104467920650638
Translational / biomarker analyses:
Kotani highlighted ctDNA findings suggesting:
- Efficacy “consistent regardless of BRAF V600E VAF at baseline”
- Resistance mutations (KRAS/NRAS/MAP2K1) “more frequent in the ENCO+CET arm than in the ENCO+CET+FOLFOX arm (41% vs. 16%)” https://x.com/DaisukeKotani/status/1978046260293857743
Key Conclusions:
In the tweet dataset, BREAKWATER is consistently interpreted as establishing front-line encorafenib + cetuximab plus chemotherapy as a new reference standard for BRAF V600E mCRC, supported by a large ORR improvement and subsequent dissemination of substantial OS and PFS gains. Ongoing discussion has moved toward (1) optimal chemotherapy backbone selection (FOLFOX vs FOLFIRI), (2) subgroup performance (e.g., early-onset CRC), and (3) resistance biology via ctDNA.
BREAKWATER Sentiments and Criticisms
Positive Reception:
Arndt Vogel, MD (#ASCO25): “Really effective, new SOC in 1st line” https://x.com/ArndtVogel/status/1928541654832525604
Cathy Eng, MD (#ASCO25): “🔵 NEW SOC 👏” and “⬆️OS 30.3M vs 15.1M (HR = 0.49)” https://x.com/CathyEngMD/status/1928544745451635178
Constructive cautions / implementation considerations:
Vogel’s summary implicitly underscored the tolerability burden by reporting high grade ≥3 AE rates with the triplet regimen (a recurrent clinical concern when intensifying first-line therapy). https://x.com/ArndtVogel/status/1928541654832525604
Eng’s early ASCO GI 2025 framing (“early trend for OS… additional data to follow”) reflects the community’s initial caution while awaiting more mature survival outcomes. https://x.com/CathyEngMD/status/1883270732987486704
Subgroup nuance (hypothesis-generating):
Eng highlighted post-hoc early-onset vs average-onset CRC differences in the original BREAKWATER trial: “Median PFS 10.9 vs. 14M” and “Median OS 23.8 vs.” (OS value truncated in the tweet text captured). https://x.com/CathyEngMD/status/2010120394511126969
Community tone:
Nicholas Hornstein, MD expressed broad enthusiasm for the molecular era in response to BREAKWATER-related discussion: “I’m so excited to be an oncologist at a time when we are just finally starting to understand the molecular and immune features of cancer!” https://x.com/GIMedOnc/status/1928454435828572175
BREAKWATER Temporal Sentiment Arc
January 2025 (ASCO GI 2025: endpoint achievement → early OS signal)
Primary/KOL tweets:
- https://x.com/CathyEngMD/status/1883270732987486704
- Tone: Optimistic and practice-forward, but with explicit “additional data to follow” language; ORR was the anchor endpoint in the discourse.
- Shift: Early positioning of the regimen as a new standard pending survival maturation.
May 2025 (ASCO 2025: maturation of OS/PFS and consolidation of “new SOC”)
Primary/KOL tweets:
- https://x.com/ArndtVogel/status/1928541654832525604
- https://x.com/CathyEngMD/status/1928544745451635178
- Tone: More definitive; survival numbers (mOS 30.3 vs 15.1; HR 0.49) became central and the “new SOC” framing strengthened.
- Shift: Conversation broadened to toxicity/feasibility (high grade ≥3 AE rates) as clinicians began translating results into real-world practice.
October 2025 (ESMO 2025: biology and resistance mechanisms)
Primary/KOL tweets:
- https://x.com/DaisukeKotani/status/1978046260293857743
- Tone: Translational and mechanistic; focus shifted from headline efficacy to ctDNA correlates and resistance mutation patterns.
- Shift: From “does the regimen work?” to “why does it work, and how does it fail?”
January 2026 (ASCO GI 2026: regimen variants, cohorts, and subgroup post-hoc work)
Primary/KOL tweets:
- https://x.com/DraMartinezLago/status/2010104467920650638
- https://x.com/OncBrothers/status/2010147873602633997
- https://x.com/CathyEngMD/status/2010120394511126969
- Tone: Optimization-focused; discussion centers on backbone selection (FOLFIRI cohort), maturity of OS, and subgroup hypotheses (early-onset CRC).
- Shift: From establishing a standard to refining it—patient selection, sequencing, and biologic monitoring.
Across presentations, BREAKWATER’s discourse progressed in a classic arc: endpoint success (ORR) → survival maturation and SOC consolidation → mechanistic ctDNA work → regimen and subgroup optimization, with toxicity and implementation considerations increasingly prominent as the regimen moved toward routine adoption.
BREAKWATER Professional Resources
