Lung Cancer
FLAURA
About the FLAURA Trial
The Phase III FLAURA trial was a double-blind study evaluating first-line osimertinib versus standard EGFR tyrosine kinase inhibitors (gefitinib or erlotinib) in patients with previously untreated EGFR-mutated advanced non-small cell lung cancer (NSCLC). The primary endpoint was progression-free survival (PFS), with overall survival (OS) as a key secondary endpoint. Osimertinib demonstrated a significant improvement in both PFS and OS, with a median OS of 38.6 months compared to 31.8 months in the comparator arm, corresponding to a hazard ratio (HR) for OS of 0.79. These results underscore the efficacy of osimertinib as a preferred frontline option in this patient population. The findings were presented at the ESMO Congress 2017 and 2019 and were discussed by key opinion leaders including Dr. Suresh S. Ramalingam and Dr. Ben Solomon, with further insights shared by Dr Riyaz Shah, Marco Tagliamento, Antonio Calles.
Table of Contents
Major Presentations and Milestones
FLAURA Trial design, results, and conclusions
FLAURA Sentiments and Criticisms
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
FLAURA Trial: Major Presentations and Milestones
Primary speakers driving the story
Initial FLAURA study schema circulated during ESMO 2017. Mature overall survival (OS) results were presented at ESMO 2019 by Suresh S. Ramalingam, MD, supporting first‑line osimertinib in EGFR‑mutant advanced NSCLC. Commentary emphasized the OS improvement over first‑generation EGFR TKIs and its practice‑changing impact.
#ESMO19 OS results from FLAURA by @RamalingamMD - primary endpoint was PFS, this is a key secondary endpoint. #OncoAlert #LCSM https://t.co/gluBDxZASy
— Stephen V Liu, MD (@StephenVLiu) September 28, 2019
7 month improvement in median OS with osimertinib compared to first gen TKI in FLAURA @RamalingamMD #ESMO19 #LCSM https://t.co/aRSyred4LV
— Nathan A. Pennell MD, PhD, FASCO (@n8pennell) September 28, 2019
FLAURA final OS; mOS 38.6m. That’s enough for me. Slam dunk. Khallas. Time to move on. #LCSM #ESMO19 https://t.co/S14sIEv97N
— Dr Riyaz Shah (@DrRiyazShah) September 28, 2019
FLAURA Trial Design, Results, and Conclusions
Trial Design:
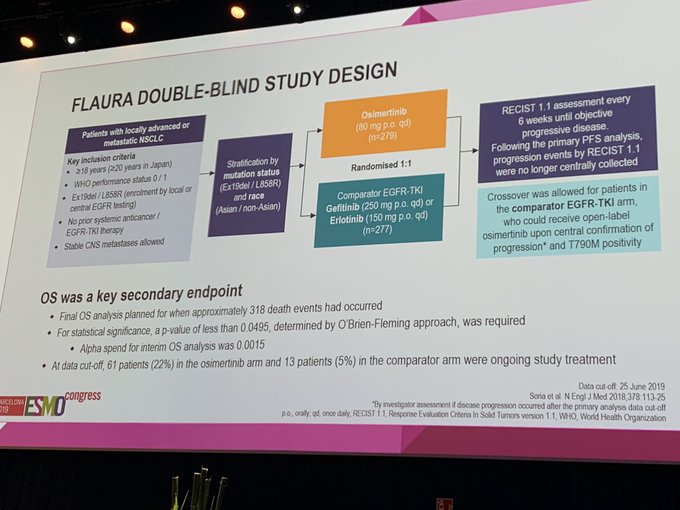
FLAURA is a phase 3, double‑blind, first‑line study in EGFR‑mutated (ex19del/L858R) advanced NSCLC, randomizing patients 1:1 to osimertinib versus gefitinib or erlotinib. The primary endpoint was progression‑free survival (PFS); overall survival (OS) was a key secondary endpoint. Eligibility included no prior EGFR‑TKI therapy; stable CNS metastases were permitted. Following primary PFS analysis, crossover to open‑label osimertinib was allowed for patients in the comparator arm with centrally confirmed progression and T790M positivity.
FLAURA study design #esmo17. Power based on mPFS 10 to 14 mo https://t.co/3AzEubmWzN
— Lecia Sequist, MD, MPH, FASCO (@LeciaSequist) September 9, 2017
Primary Results:
At ESMO 2019, investigators reported an OS improvement with first‑line osimertinib versus first‑generation TKIs. KOLs summarized median OS for osimertinib as 38.6 months and a ~7‑month median OS advantage versus comparator in the final analysis. PFS remained the primary endpoint; OS was prespecified as a key secondary endpoint.
Key Conclusions:
First‑line osimertinib improves survival outcomes versus gefitinib/erlotinib in EGFR‑mutated advanced NSCLC, consolidating its role as standard of care. Discussion has since shifted to optimization strategies (e.g., combination regimens) and to understanding subgroup differences and CNS disease considerations noted in exploratory analyses.
FLAURA Sentiments and Criticisms
Positive Reception:
Dana-Farber News (Pasi A. Jänne, MD, PhD): "Dana-Farber's Pasi A. Jänne, MD, PhD, comments on the practice changing results from the FLAURA study, which firmly established osimertinib as front-line therapy in patients with EGFRm nonsmall cell lung cancer #ESMO19 #NSCLC #lcsm" https://x.com/DanaFarberNews/status/1178658399602593798
Joshua Bauml, MD: "Given early separation of curves between osimertinib and gefitinib/erlotinib in FLAURA, I wonder if osimertinib could overcome the need for concurrent chemotherapy. Interesting study, but will need to be validated with osimertinib before I change my practice #LCSM #ASCO18" https://x.com/Jbauml/status/1003755952833814528
Critical Perspectives and Nuance:
@TonyRen_HK @EMA_News The very first time I see non-Asians do better than Asians in targeted therapy. Weird, right?
— Dr. Antonio Calles 🧡💀 (@Tony_Calles) September 28, 2019
@Tony_Calles @TonyRen_HK @EMA_News Not exactly non-Asians doing better than Asians. Asians did just as well with first gen EGFR TKIs as with osimertinib. Non-Asians did better w/osimertinib. Could be diff in biology, diffs in health care systems where pts were treated, or some combo of these factors.
— H. Jack West, MD, FASCO (@JackWestMD) September 29, 2019
Patrick C. Ma, MD raised an alternative hypothesis: "Quite intriguing data. Wonder if it has to do with CNS mets difference btw the two groups: Asian vs Non-Asian... 🤔" https://x.com/PatrickCMa1/status/1178144095530835970
Joshua Bauml, MD added perspective on outcomes magnitude: "That being said 52.2 months is pretty impressive overall survival in this study #LCSM #ASCO18" https://x.com/Jbauml/status/1003756715962191872
FLAURA Temporal Sentiment Arc
2017 (Design Preview at ESMO17)
Primary/KOL tweets:
- https://x.com/LeciaSequist/status/906540878650646528
- Tone: Focus on study schema and powering assumptions for PFS.
- Shift: Anticipation for randomized efficacy results and CNS‑relevant design elements.
2018 (Early Community Interpretation)
- https://x.com/Jbauml/status/1003755952833814528
- https://x.com/Jbauml/status/1003756715962191872
- Tone: Enthusiasm for early curve separation with osimertinib; open questions about whether chemotherapy is needed concurrently.
- Shift: Awaiting mature OS to confirm durability and inform practice change.
2019 (ESMO19 Final OS Analysis)
Primary/KOL tweets:
#ESMO19 OS results from FLAURA by @RamalingamMD - primary endpoint was PFS, this is a key secondary endpoint. #OncoAlert #LCSM https://t.co/gluBDxZASy
— Stephen V Liu, MD (@StephenVLiu) September 28, 2019
- https://x.com/n8pennell/status/1177980420002066432
- https://x.com/DrRiyazShah/status/1177980452877082627
- https://x.com/DanaFarberNews/status/1178658399602593798
- Tone: Strong support for first‑line osimertinib with median OS improvement and practice‑changing implications.
- Shift: Attention to subgroup differences (Asian vs non‑Asian) and CNS disease as potential modifiers.
2024–2025 (Optimization Era: Combination Strategies and Next‑Line Integration)
- https://twitter.com/StephenVLiu/status/1753717500754235480
- https://twitter.com/CharuAggarwalMD/status/1759614268951896498
- Tone: Acknowledgment that most patients progress within ~2 years on osimertinib monotherapy; exploration of combinations (e.g., FLAURA2 chemotherapy add‑on, MARIPOSA amivantamab) with improved PFS but added toxicity, and questions about OS benefit.
- Shift: Integration across disease settings (e.g., adjuvant ADAURA, LAURA in unresectable stage III) and alignment of sequencing to maximize long‑term survival.
Overall, discourse progressed from design and early efficacy signals to definitive OS improvement establishing first‑line osimertinib, followed by a forward‑looking focus on combination strategies, subgroup nuances, and CNS considerations to extend benefit.
FLAURA Professional Resources
