The AQUILA trial is a Phase 3 randomized study evaluating daratumumab (brand name DARZALEX®, manufactured by Janssen Biotech, Inc., a Johnson & Johnson company) versus active monitoring in patients with high-risk smoldering multiple myeloma (SMM). Involving 390 patients and a median follow-up of 5.5 years, the study demonstrated that daratumumab significantly prolonged progression-free survival (63.1% vs. 40.8%) and showed a favorable trend in overall survival (93% vs. 87%) compared to monitoring alone. Daratumumab was well tolerated, with no major quality of life impairment reported. These results, presented at ASH 2024 by Dr. Meletios Dimopoulos, suggest daratumumab may play a pivotal role in delaying progression in high-risk SMM.
Smoldering Myeloma
AQUILA
About the AQUILA Trial
Table of Contents
Major Presentations and Milestones
AQUILA Trial design, results, and conclusions
AQUILA Sentiments and Criticisms
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
HISTORIC AND PARADIGM CHANGING:
— Vincent Rajkumar (@VincentRK) November 6, 2025
FDA approves daratumumab for high risk smoldering multiple myeloma based on the AQUILA trial— the FIRST ever treatment approved for this indication.
Excellent news and thanks to @JNJInnovMed and the huge worldwide team of investigators for… pic.twitter.com/CgmwdzqmAE
AQUILA Trial: Major Presentations and Milestones
Primary speakers driving the story
ASH 2024: Primary analysis dissemination and rapid KOL interpretation.
Source: https://x.com/VincentRK/status/1866205046855377366
NEJM publication (May 2025): Publication milestone and circulation of key endpoints.
Source: https://x.com/NEJM/status/1920463768926237132
ASH 2025 cycle (“#ASH25”): Follow-on discussion emphasizing risk stratification and “treat vs observe” implementation pathways.
Source: https://x.com/VincentRK/status/1993402192103956908
AQUILA Trial Design, Results, and Conclusions
Trial Design:
- Phase 3, randomized 1:1; N=390; enrollment Dec 2017–May 2019 across 124 sites in 23 countries.
- Population: high-risk smoldering multiple myeloma.
- Arms: daratumumab subcutaneous monotherapy (fixed duration) vs active monitoring.
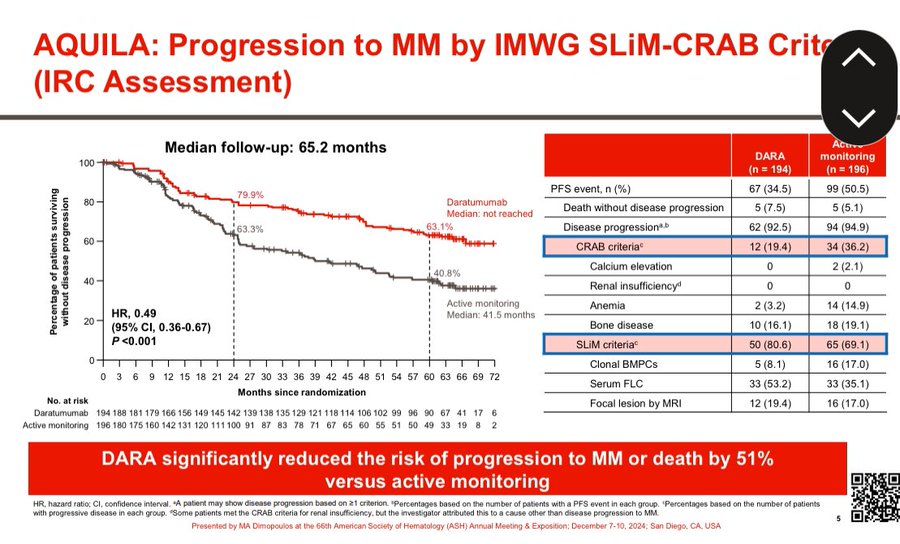
- Primary endpoint: PFS by independent review committee per IMWG SLiM-CRAB criteria.
- Key secondary endpoints: ORR, time to first-line treatment for MM, PFS on first-line treatment for MM, overall survival.
Primary Results:
- PFS: HR 0.49 (95% CI 0.36–0.67).
- Overall survival: HR 0.52 (95% CI 0.27–0.98); 5-year OS 93.0% vs 86.9%; deaths 15 (7.7%) vs 26 (13.3%).
- PFS on first-line treatment for MM: HR 0.58 (95% CI 0.35–0.96).
- Subgroup highlight: retrospective Mayo 2018 criteria PFS HR 0.36 (95% CI 0.23–0.58).
Source: https://x.com/OncBrothers/status/1866529170832101849
Additional published endpoint (as stated by NEJM):
- 5-year PFS: 63.1% (daratumumab monotherapy) vs 40.8% (active monitoring).
Source: https://x.com/NEJM/status/1921973720434016868
Safety:
- Any-grade TEAEs: 96.9% vs 82.7%.
- Grade 3–4 TEAEs: 40.4% vs 30.1%.
- Serious TEAEs: 29.0% vs 19.4%.
- Grade 5 TEAEs: 1.0% vs 2.0%.
- Treatment discontinuation due to TEAE (daratumumab arm): 11 (5.7%).
- Selected grade 3–4 TEAEs (≥5%): COVID-19 TEAEs 8.8% vs 5.1%; hypertension 5.7% vs 4.6%.
Source: https://x.com/victorshiyulin/status/1866273872569679896
Key Conclusions:
- Fixed-duration daratumumab in high-risk smoldering myeloma improved PFS and was associated with improved overall survival versus active monitoring.
- Safety profile included higher rates of grade 3–4 and serious TEAEs versus active monitoring, with discontinuation due to TEAE reported at 5.7% in the daratumumab arm.
AQUILA Sentiments and Criticisms (Themes)
Theme 1 — Practice-changing framing vs observe-first implementation
- Rajkumar (practice-changing framing): “Just out: Paradigm changing AQUILA randomized trial…”
Source: https://x.com/VincentRK/status/1866205046855377366
- Rajkumar (ASH25 observation pathway): “How to follow High Risk Smoldering Myeloma: Observation…”
Source: https://x.com/VincentRK/status/1993402192103956908
- Chakraborty (observe-first stance): “...but I would still do active surveillance. Reassuring to see that almost no morbid progressions in either arm.”
Source: https://x.com/rajshekharucms/status/1866277618653581388
Theme 2 — OS signal interpretation and control-arm subsequent therapy
- Prasad (multiplicity concern): “OS doesn't report how it adjust for multiple looks…”
Source: https://x.com/VPrasadMDMPH/status/1866216887597883579
- Goodman (post-progression CD38 exposure question): “How many patients in control got Daratumumab on progression”
Source: https://x.com/AaronGoodman33/status/1865044832928043469
- Chakraborty (modern-era regimen critique): “...~30% of patients received a clearly suboptimal 1st line… which makes the OS signal unreliable…”
Source: https://x.com/rajshekharucms/status/1866205353408659948
Theme 3 — Risk definition, SLiM events, and overtreatment risk
- Rajkumar (ODAC/overtreatment): “I’m not one who likes over treating…”
Source: https://x.com/VincentRK/status/1924950447217090582
- Chakraborty (SLiM event relevance evolving): “...risk of progression to CRAB myeloma is much lower…”
Source: https://x.com/rajshekharucms/status/1924916232295190619
- Chakraborty (overtreatment framing): “~40% of patients in the control arm… did not require start… at 5 years!”
Source: https://x.com/rajshekharucms/status/1990126470841164022
Theme 4 — Imaging sensitivity and baseline diagnostic purity
- Lentzsch: “...interesting to see the HR if pt would have received DWI-WBMRI to exclude active MM”
Source: https://x.com/SLentzsch/status/1866205992498913281
- Chakraborty (protocol detail): “As per protocol, MRI spine/pelvis was performed at baseline (not WB DW-MRI).”
Source: https://x.com/rajshekharucms/status/1866206213685567724
Theme 5 — Safety burden and fixed-duration tradeoffs
- Rajkumar: “There is a 10% absolute higher risk of grade 3-4 AEs… related to longer period on study…”
Source: https://x.com/VincentRK/status/1866205079893942357
AQUILA Temporal Sentiment Arc
ASH 2024: primary analysis release and immediate positioning
- Rajkumar “paradigm changing” thread lead: https://x.com/VincentRK/status/1866205046855377366
- Derman trial framing: https://x.com/bdermanmd/status/1866255928443769129
- Prasad early critique: https://x.com/VPrasadMDMPH/status/1866216887597883579
2025: publication and regulatory/practice integration
- NEJM publication announcement: https://x.com/NEJM/status/1920463768926237132
- NEJM endpoint dissemination (5-year PFS): https://x.com/NEJM/status/1921973720434016868
- ODAC discussion (overtreatment/refinement): https://x.com/VincentRK/status/1924950447217090582
- NCCN Category 1 signal: https://x.com/VincentRK/status/1937859797379871065
ASH 2025 cycle: risk stratification and “treat vs observe” implementation
- Rajkumar monitoring pathway (observation): https://x.com/VincentRK/status/1993402192103956908
- Rajkumar ASH25 follow-on (IMWG 2020 stratification): https://x.com/VincentRK/status/1996293530948362598
- Chakraborty (ASH25-timed “do not routinely recommend treatment yet”): https://x.com/rajshekharucms/status/1996947659580469438
AQUILA Professional Resources
