Lung Cancer
FLAURA2
FLAURA2 Trial Top Tweets
FDA has no standards
— Vinay Prasad MD MPH (@VPrasadMDMPH) February 16, 2024
Approving chemo + Osi based on FLAURA2 is a terrible decision
Osi -> chemo may have similar or better OS with better QoL.
FDA has no clue. They are permitting an option that could worsen outcomes for people.
Terrible decision, par for course pic.twitter.com/bMgYZk6W9T
FLAURA2 now FDA approved. It’s the biggest change in this space for some years. I’ll be fascinated to see how this gets used. All comers? Brain mets predominantly? Many patients welcome the opportunity to avoid chemo. https://t.co/gCBxma6XmP
— Dr Riyaz Shah (@DrRiyazShah) February 17, 2024
Dr. @dplanchard at #WCLC25 with highly anticipated Presidential Plenary presentation with OS results from FLAURA2: first line chemo + osimertinib improves OS from 37.6 to 47.5m, HR 0.77, 4y OS rate 41% to 49%. Benefit seen across subgroups. Strong results - similar to MARIPOSA. pic.twitter.com/qYOP287NyK
— Stephen V Liu, MD (@StephenVLiu) September 7, 2025
EGFR update
— Benjamin Besse (@BenjaminBesseMD) September 7, 2025
7 potential options:
•3rd gen TKI: osimertinib, lazertinib, aumolertinib
•Amivantamab
•Pemetrexed
•Carboplatin
•Ivonescimab
•Dato-DXd
OS data favor combos upfront—but real-world ≠ trial. In RWD, ~40% of newly diagnosed pts wouldn’t qualify for FLAURA2 #WCLC25 pic.twitter.com/U08kwhQ5Yg
About the FLAURA2 Trial
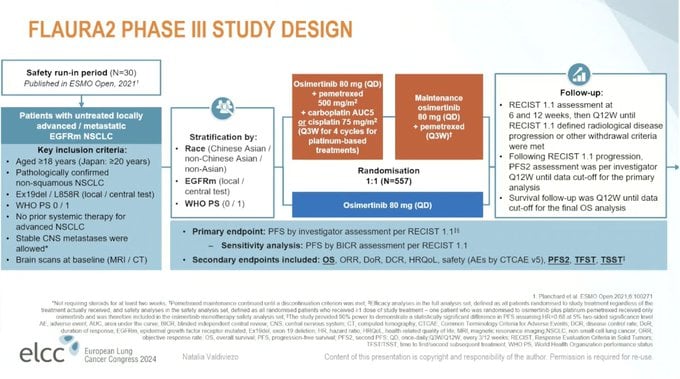
The FLAURA2 trial is a Phase III, randomized study investigating the efficacy of osimertinib in combination with chemotherapy compared to osimertinib monotherapy in patients with previously untreated, locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring EGFR-sensitizing mutations (Ex19del or L858R). Eligible patients had not received prior systemic therapy for advanced disease. The primary endpoint was progression-free survival (PFS) assessed by investigators, with secondary endpoints including overall survival (OS), objective response rate (ORR), duration of response (DoR), and safety. The combination of osimertinib plus chemotherapy resulted in improved PFS over osimertinib alone, with an improved OS. While the combination arm had a higher incidence of adverse events—primarily hematologic toxicities—the safety profile remained manageable. These findings were presented at the European Lung Cancer Congress (ELCC) 2024, with expert commentary provided by Dr. Natalia Valdiviezo and shared by Dr. Stephen V. Liu. Additional insights into the trial’s design, efficacy, and safety outcomes were shared via social media by Dr. Liu and Dr. Martin Dietrich
Trial Methodology
Study Design: Phase III, randomized, multi-center trial
Population: Patients with EGFR-mutated advanced NSCLC who had not previously been treated
Interventions: Randomization to receive either osimertinib plus chemotherapy or osimertinib alone
Endpoints: Primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS) and safety assessment.
Post-progression outcomes including OS from FLAURA-2 at ELCC24. Adding platinum-based chemotherapy to first-line osimertinib for EGFR NSCLC improved PFS (HR 0.62).Adding chemotherapy improved time to first and second subsequent therapy - which was primarily chemotherapy++ pic.twitter.com/Y5Acy8v3NT
— Excellence in Oncology Care - EIOC (@EiocOncology) March 22, 2024
Dr. Natalia Valdiviezo presents post-progression outcomes including OS from FLAURA-2 at #ELCC24. Adding platinum-based chemotherapy to first-line osimertinib for #EGFR NSCLC improved PFS (HR 0.62) but what is the impact on long-term outcomes? pic.twitter.com/gIXQrFOltq
— Stephen V Liu, MD (@StephenVLiu) March 21, 2024
Detailed Results
Progression-Free Survival (PFS)
-
Combination Therapy (Osimertinib + Chemo): Demonstrated an improved median PFS of 25.5 months compared to 16.7 months with osimertinib alone.
Overall Survival (OS)
-
Following the AstraZeneca announcement the Overall Survival data was presented by Dr. Planchard at the #WCLC25 meeting. The Combination of osimertinib and chemo improved mOS 47.5m v 37.6m with single agent osimertinib (HR 0.77 p=0.02).
FLAURA-2: Osimertinib + chemo significantly improves OS in EGFR-mutated advanced lung cancer.
— Yakup Ergün (@dr_yakupergun) July 21, 2025
After MARIPOSA, another option with positive OS data.
Current question: MARIPOSA vs FLAURA-2🤔https://t.co/NJy3RORUQP pic.twitter.com/EbJvB03aHF
#WCLC25 Presidential
— Jarushka Naidoo (@DrJNaidoo) September 7, 2025
FLAURA2 OS in 1L EGFR+ NSCLC:
- mOS 47.5m v 37.6m (HR 0.77 p=0.02)
- duration of osi 30.5m on osi+chemo v 21.2m osi- combo delays acq resistance? - 72% got 2L chemo- does this add ~9m?
- all subgrps benefit (?BM, ?co-mtns)
Congrats @dplanchard @iaslc #LCSM pic.twitter.com/8INwNH7W6Y
Safety and Tolerability
The addition of chemotherapy increased toxicity, including grade 3/4 adverse events, compared to osimertinib monotherapy.
Clinical Significance
The FLAURA2 trial introduces a potential new standard of care by demonstrating improved efficacy of osimertinib in combination with chemotherapy for EGFR-mutated NSCLC, particularly benefiting patients with high-risk features such as CNS involvement. However, the increased toxicity associated with the combination regimen necessitates careful patient selection and shared decision-making regarding treatment options.
Dr. @PatelOncology discusses FLAURA2 and consideration of intensification regimens though still under investigation
— Jennifer A. Marks, MD (@jennifermarksmd) June 25, 2025
🫁molecular risk (Rb, p53, ctDNA+)
🧠clinical factors (CNS metastasis) #DAVAlung #lcsm #lcam @EGFRResisters #HawaiiLung25 pic.twitter.com/a9vhZ7Kf7t
FLAURA2 TRIAL
KOL Sentiment Table
KOL |
Pulse Score |
|---|---|
 Dr. Stephen Liu |
49 |
 Dr. Eric Singhi |
40 |
 Dr. Rami Manochakian |
29 |
 Dr. Santhosh Ambika |
26 |
 Dr. Jarushka Naidoo |
23 |
 Oncology Brothers |
20 |
 Dr. Balazs Halmos |
19 |
 Dr. Joshua Reuss |
18 |
 Dr. Estelamari Rodriguez |
18 |
 Dr. Patrick Forde |
17 |
 Dr. Charu Aggarwal |
14 |
 Dr. Vinayak Prasad |
13 |
 Dr. Aakash Desai |
12 |
 Dr. Bijoy Telivala |
10 |
 Dr. Chandler Park |
10 |
 Dr. Nathan Pennell |
9 |
 Dr. Tejas Patil |
9 |
 Dr. Kelsey Pan |
9 |
 Dr. David Camidge |
9 |
 Dr. Howard West |
8 |
 Dr. Luis Raez |
8 |
Dr. Clay Reed |
7 |
 Dr. Christian Rolfo |
6 |
 Dr. Sandip Patel |
6 |
 Dr. Kyaw Thein |
5 |
 Dr. Chul Kim |
5 |
 Dr. Thomas Varghese |
5 |
 Dr. Gilberto Lopes |
5 |
 Dr. Michael Pishvaian |
5 |
 Dr. Misty Shields |
4 |
 Dr. Julia Rotow |
4 |
 Dr. Tisdrey Torres |
3 |
 Dr. Narjust Florez |
3 |
 Dr. Brendon Stiles |
3 |
 Dr. Ana Velazquez Manana |
3 |
 Dr. Fawzi Abu Rous |
3 |
 Dr. Sewanti Limaye |
3 |
 Dr. Isabel Preeshagul |
2 |
 Dr. Sarah Waliany |
2 |
 Dr. Susan Scott |
2 |
 Dr. Suresh Ramalingam |
2 |
 Dr. Jeffrey Ryckman |
2 |
 Dr. Martin Dietrich |
2 |
 Dr. Anis Toumeh |
2 |
 Dr. Xiuning Le |
2 |
 Dr. Sean Mcbride |
2 |
 Dr. Dipesh Uprety |
2 |
 Dr. Aditya Juloori |
2 |
 Dr. Petros Grivas |
2 |
 Dr. Marina Garassino |
2 |
 Dr. Jonathan Spicer |
2 |
 Dr. Patrick Ma |
2 |
 Dr. Coral Olazagasti |
2 |






