Breast Cancer
PATINA
About the PATINA Trial
The PATINA trial was a Phase 3, randomized, open-label study evaluating the addition of palbociclib to standard anti-HER2 therapy and endocrine therapy in patients with HER2-positive, hormone receptor-positive metastatic breast cancer. The trial’s primary endpoint was progression-free survival (PFS), with secondary endpoints including overall survival (OS) and safety. The addition of palbociclib significantly improved PFS compared to anti-HER2 and endocrine therapy alone, supporting the strategy of incorporating CDK4/6 inhibition into the treatment of this molecular subtype. The safety profile was manageable and consistent with previously reported data on palbociclib, with no unexpected adverse events. These findings were presented at the 2024 San Antonio Breast Cancer Symposium (SABCS) and were highlighted by key opinion leaders such as Ilana Schlam and the Oncology Brothers, who provided further insights into the clinical implications for integrating CDK4/6 inhibitors in HER2+/HR+ metastatic disease.
Table of Contents
Major Presentations and Milestones
PATINA Trial design, results, and conclusions
PATINA Sentiments and Criticisms
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
FDA approval signal (tweeted): Erika P. Hamilton, MD noted: “T-DXd + pertuzumab approved for 1st L HER-2+ #bcsm!” and framed the downstream clinical question as “debates around induction length, maintenance, re-induction etc.” https://x.com/ErikaHamilton9/status/2000621940491657511
PATINA Trial: Major Presentations and Milestones
Primary speakers driving the story
Online discussion around PATINA was catalyzed by Paolo Tarantino, MD (who signposted the trial’s late addition to the San Antonio program) and then rapidly consolidated around Otto Metzger, MD (Dana-Farber Cancer Institute), who presented the phase 3 results at the San Antonio Breast Cancer Symposium (SABCS 2024). The early narrative emphasized a “practice-changing” maintenance strategy in HR+/HER2+ metastatic breast cancer after induction THP, with multiple KOLs highlighting the absolute PFS gain (≈15 months) and the practical implications for sequencing in the emerging DESTINY-Breast09 era.
‼️When you thought you had the #SABCS24 program figured out, here’s the PRACTICE-CHANGING #PATINA trial being added!
— Paolo Tarantino (@PTarantinoMD) Dec 12, 2024
For the impatient ones, press release here: https://t.co/C3HIxbSnRb
If you’re able, though, make sure to attend the data presentation today by @Otto_DFCI! #bcsm https://t.co/ozEzYaw3gQ
As the presentation landed, Sara Tolaney, MD (Dana-Farber Cancer Institute) posted a concise summary of the key efficacy results and follow-up, reinforcing how quickly the community interpreted the trial as actionable for routine care.
PATINA: 1L maintenance HP +ET +/- palbo in ER+ HER2+ BC
— Sara Tolaney (@stolaney1) Dec 12, 2024
n=518
72% had prior tx for early-stage dz
PFS 44.3 vs 29.1 mo, HR 0.74, p=0.0074
median f/u 52.6 mo
OS 74% vs 29.8% at 5 yrs
Amazing benefit!!!! Congrats to @Otto_DFCI + team!
#SABCS24 @oncoalert @DFCI_BreastOnc
By SABCS 2025, the conversation broadened from “PFS magnitude” to CNS outcomes and implementation in a rapidly shifting 1L HER2+ landscape (DB-09, HER2CLIMB-05, and PATINA). Stephanie Graff, MD, FACP, FASCO highlighted the CNS progression signal with palbociclib.
PATINA demonstrates lower rates of CNS progression in both all pts and those with brain meta at baseline with the addition of palbociclib. @Otto_DFCI @OncoAlert #SABCS25 https://t.co/0tlUorXaIG
— Stephanie Graff, MD, FACP, FASCO (@DrSGraff) Dec 11, 2025
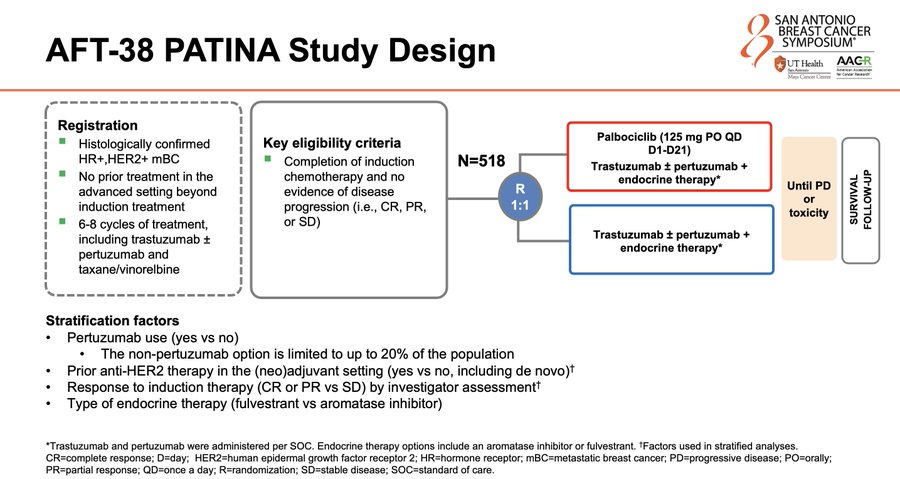
PATINA Trial Design, Results, and Conclusions
Trial Design:
PATINA (AFT-38) is a phase 3 trial in HR+/HER2+ metastatic breast cancer evaluating whether adding palbociclib to standard anti-HER2 plus endocrine maintenance improves outcomes after induction therapy. As summarized by Ilana Schlam, MD, patients (n=518) received induction THP and then were treated with HP + endocrine therapy ± palbociclib. https://x.com/IlanaSchlam/status/1867267431405678599
Primary Results (PFS):
Multiple KOLs converged on the same core efficacy message: a clinically large improvement in PFS with palbociclib added to maintenance anti-HER2 + endocrine therapy. Paolo Tarantino, MD quoted: “44 vs 29 months, HR 0.74, p=0.007.” https://x.com/PTarantinoMD/status/1867270082625196056
Ilana Schlam, MD contextualized the magnitude and population: “PFS was 29 vs 44 months with palbo” in “n=518 HR+/HER2+ mBC” after THP induction. https://x.com/IlanaSchlam/status/1867267431405678599
Erika Hamilton, MD emphasized the absolute delta and maintenance framing: “Wow, #PATINA shows 15 month improved PFS … when palbociclib is added to ET +HER-2 antibodies in maintenance 1st line setting after chemo induction.” https://x.com/ErikaHamilton9/status/1867234802593849710
Overall Survival (immature):
Community posts repeatedly cautioned that OS was not yet definitive. Oncology Brothers summarized: “OS not mature yet.” https://x.com/OncBrothers/status/1867296019551015341
CNS progression (SABCS 2025 focus):
At SABCS 2025, discussion increasingly centered on whether palbociclib might reduce CNS progression risk. Sara Tolaney, MD wrote: “Among pts w/no CNS mets at baseline, cumulative risk of CNS progression or death: 12.8% (palbo) vs 19.0% (control), p=0.0378 … caveat: no mandatory CNS imaging at baseline or in f/u.” https://x.com/stolaney1/status/1999247544233115957
Nicole Casasanta, MD provided additional CNS detail and a baseline-CNS subgroup snapshot: “At 36 mo, cum. incidence CNS progression/death 13.4% vs 19.9% for palbo vs control P =0.04,” while noting small baseline CNS numbers (“Palbo (n=11), Control (n=9)”). https://x.com/ncasasanta/status/2001099600392802502
Patient-reported outcomes / QoL:
Later in the evidence arc, PROs were emphasized as reassuring for long-term maintenance. Guilherme Nader Marta, MD posted that adding palbociclib “maintained HRQoL,” with “similar time to symptom progression (HR 0.89)” and stable FACT-B TOI and EQ-5D-5L scores over time. https://x.com/GuiNaderMarta/status/1980197881597534349
Key Conclusions:
Across tweets, PATINA was interpreted as establishing a compelling maintenance strategy after induction THP in HR+/HER2+ metastatic disease, with a consistent ~15-month median PFS improvement and emerging (but methodologically caveated) CNS signals. The practical clinical question shifted toward how to integrate PATINA-style maintenance into a first-line environment increasingly influenced by antibody–drug conjugates (e.g., T-DXd + pertuzumab) and tucatinib-based maintenance trials.
PATINA Sentiments and Criticisms
Positive Reception:
Paolo Tarantino, MD (SABCS 2024): “Otto Metzger (@Otto_DFCI) presents the practice-changing results from the #PATINA phase 3 trial… significantly and meaningfully improved PFS (44 vs 29 months, HR 0.74, p=0.007). 44 months!!!!!!!!!!!!!!!!!!!!!!!!” https://x.com/PTarantinoMD/status/1867270082625196056
Sara Tolaney, MD (SABCS 2024): “PFS 44.3 vs 29.1 mo, HR 0.74, p=0.0074… Amazing benefit!!!!” https://x.com/stolaney1/status/1867267861036904636
Hope Rugo, MD (SABCS 2024 anticipation): “Long awaited results and very exciting advance for our patients!! Almost all of my patients still on this well tolerated quadruplet Rx.” https://x.com/hoperugo/status/1867227709312504307
Critical Perspectives / Implementation Concerns:
Enrique Soto (SABCS 2024): “Pts have gotten through a tough chemo, if you add something toxic to their subsequent therapy, it should increase OS! … We NEED to stop saying toxicities are ‘manageable’, the palbo group had much more toxicities, it should be said like that, without spin” https://x.com/EnriqueSoto8/status/1867269383703474600
Suyog Cancer (commentary on design/selection): “So that is how smart trial design help you to get impressive PFS results. You select pts with good biology at the end of induction chemo…” https://x.com/SuyogCancer/status/1867393684821447139
Anis Toumeh, MD (ASCO 2025 context, comparing to evolving T-DXd strategies): “With PATINA mPFS of 44m, I am not sure this is straightforward from a change of practice standpoint.” https://x.com/AnisToumeh/status/1929542709519249891
Nuanced consensus emerging:
Even among supportive voices, the debate moved quickly to duration and tolerability of prolonged T-DXd-based induction and the need for maintenance. Erika Hamilton, MD asked: “do we think we are really going to give years and years of TDXd 1st line? … Feel like we should have a maintenance strategy after induction.” https://x.com/ErikaHamilton9/status/1867238639362879729
Eleonora Teplinsky, MD, FASCO (SABCS 2025): “Not everyone needs upfront T-DXd and the side effects of prolonged T-DXd are significant. A maintenance approach is critical. THP with maintenance tucatinib or palbociclib is an excellent option.” https://x.com/drteplinsky/status/1998803333126267270
PATINA Temporal Sentiment Arc
2024 (SABCS24: “practice-changing” reveal)
Primary/KOL tweets:
Otto Metzger (@Otto_DFCI) presents the practice-changing results from the #PATINA phase 3 trial: adding palbociclib to maintenance ET after 1L THP for HR+/HER2+ MBC significantly and meaningfully improved PFS (44 vs 29 months, HR 0.74, p=0.007).
— Paolo Tarantino (@PTarantinoMD) Dec 12, 2024
44 months!!!!!!!!!!!!!!!!!!!!!!!! https://t.co/a1DT4eS3B8
- https://x.com/stolaney1/status/1867267861036904636
- https://x.com/IlanaSchlam/status/1867267431405678599
- https://x.com/ErikaHamilton9/status/1867234802593849710
- https://x.com/EnriqueSoto8/status/1867269383703474600
- Tone: High enthusiasm around a large absolute PFS gain, paired with immediate clinician-level questions about toxicity framing and whether OS would ultimately confirm the value proposition.
- Shift: From “headline PFS” to early scrutiny of trial design (post-induction selection) and the need for OS maturation.
2025 (ASCO25/ESMO25: sequencing debates and QoL data)
Primary/KOL tweets:
- https://x.com/PTarantinoMD/status/1927120376728846341
- https://x.com/AnisToumeh/status/1929542709519249891
- https://x.com/GaiaGriguolo/status/1929521120597688539
- https://x.com/GuiNaderMarta/status/1980197881597534349
- Tone: More cautious and comparative—PATINA is increasingly discussed as a maintenance “module” to be integrated into ADC-era pathways (e.g., DB-09), with attention to PRO/QoL and real-world feasibility.
- Shift: From “is it practice-changing?” to “where does it fit?” (induction length, re-induction, and how to rationally sequence ET, HER2 therapy, and ADCs).
Late 2025 (SABCS25: CNS protection and maintenance strategy consolidation)
Primary/KOL tweets:
- https://x.com/DrSGraff/status/1999248636291473716
- https://x.com/stolaney1/status/1999247544233115957
- https://x.com/GaiaGriguolo/status/1999247505905844506
- https://x.com/ncasasanta/status/2001099600392802502
- Tone: Strong interest in CNS endpoints as a differentiator, with explicit methodological caveats (non-mandated CNS imaging) and increasing emphasis on maintenance as the practical “bridge” after induction (whether THP or T-DXd+P).
- Shift: From PFS-centric interpretation to CNS outcomes + implementation science (who needs prolonged ADC, who can de-escalate, and how ET impacts benchmarks).
Across the full arc, PATINA evolved from a SABCS 2024 “late-breaking practice change” story into a 2025–2026 implementation and sequencing debate: how to preserve long PFS while minimizing cumulative toxicity, and whether palbociclib-containing maintenance meaningfully changes CNS natural history in HR+/HER2+ metastatic disease.
PATINA Professional Resources
