Lung Cancer
ADRIATIC
About the ADRIATIC Trial
The ADRIATIC trial is a Phase III, randomized, double-blind study evaluating the role of durvalumab ± tremelimumab as consolidation therapy in patients with limited-stage small cell lung cancer (LS-SCLC) who have completed concurrent chemoradiotherapy (cCRT). Patients were randomized to receive durvalumab, durvalumab plus tremelimumab, or placebo following cCRT. The co-primary endpoints were overall survival (OS) and progression-free survival (PFS). Durvalumab monotherapy demonstrated a statistically significant improvement in both OS (hazard ratio [HR] 0.73; p=0.0104) and PFS (HR 0.76; p=0.0161) compared to placebo. These benefits were consistent across predefined clinical subgroups. The findings were presented at the 2024 ASCO Annual Meeting by Dr. David R. Spigel.
Table of Contents
Major Presentations and Milestones
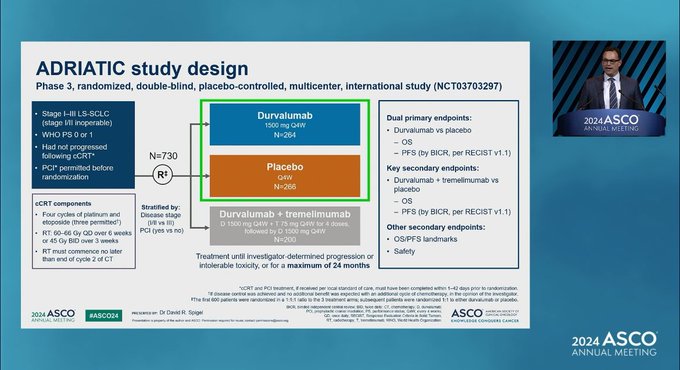
ADRIATIC Trial design, results, and conclusions
ADRIATIC Sentiments and Criticisms
ADRIATIC Temporal Sentiment Arc
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
ADRIATIC Trial: Major Presentations and Milestones
Primary speakers driving the story: via Dr. Ryaz Shah from ASCO24, "ADRIATIC. A brilliant presentations by @DavidRSpigel and discussion by @LaurenByersMD..."
ADRIATIC. A brilliant presentation by @DavidRSpigel and discussion by @LaurenByersMD. I really reflected on the comment about despite similar HR's, the absolute extensions are massive in LSvES. @BTOGORG #ASCO24 pic.twitter.com/zWWN7iTdAb
— Dr Riyaz Shah (@DrRiyazShah) June 2, 2024
Great discussion of IO in SCLC by @LaurenByersMD @ASCO after presentation of ADRIATIC consolidation durvalumab after chemoXRT in LS-SCLC by @DavidRSpigel #MedIQASCO2024 pic.twitter.com/jGpmaHq03w
— Sandip Patel MD FASCO (@PatelOncology) June 2, 2024
Jarushka Naidoo, MD (ASCO 2024 plenary live coverage): “#ASCO24 Plenary🔥 Ph III ADRIATIC trial of consolidation Durva +/-Treme post cCRT for LS SCLC: - benefit in mOS 55.9 v 33.4m (HR 0.73, p=0.01) & mPFS 16.6 v 9.2m … First advance in 30+yrs in LS SCLC” https://twitter.com/DrJNaidoo/status/1797369436430594290
A/Prof Shankar Siva, MD, PhD (ASCO 2024 plenary; radiation oncology perspective): “pivotal ADRIATIC ph III … durvalumab after chemoradiotherapy (cCRT) ± PCI … ⬆️ OS (HR 0.73), median OS 55.9 vs 33.4. Grade 3/4 AEs 24.3% vs 24.2%. Practice changing!!!” https://twitter.com/_ShankarSiva/status/1797367226405724564
Stephen V. Liu, MD (thoracic oncology; discussant takeaways): “Dr. @LaurenByersMD with a wonderful discussion of ADRIATIC at #ASCO24 – highlighting a much greater impact on OS with immunotherapy in the limited stage setting than seen in extensive stage.” https://twitter.com/StephenVLiu/status/1797369428226494869
KOL reactions to the OS presentation and its significance
Noemi Reguart, MD: “Second practice changing trial of the day… OS/PFS meet (3-y FUP): mOS 55.9 vs 33.4 (HR 0.73) and 24% reduction in risk of PFS/death (16.6 vs 9.2 mo; HR 0.76)” https://twitter.com/NReguart/status/1797369372844994874
Nathan A. Pennell, MD, PhD, FASCO: “Standing ovations as KM curves revealed… practice changing!” https://twitter.com/n8pennell/status/1797369975868367054
Standing ovations as KM curves revealed interrupt talks for both speakers for LAURA and ADRIATIC trials at #ASCO24 plenary. Totally worth it to be here in person to experience practice changing! pic.twitter.com/iHuCPjczpg
— Nathan A. Pennell MD, PhD, FASCO (@n8pennell) June 2, 2024
Salma Jabbour, MD: “ADRIATIC study is the new standard of care for limited stage small cell lung cancer… improved mOS to 56 mo from 33 mo (placebo) and mPFS of 17 months vs 9 mo” https://twitter.com/SalmaJabbour1/status/1797369378771448318
Erika Hamilton, MD: “So proud of @DavidRSpigel for an excellent presentation… transforming results… Big win for patients!” https://twitter.com/ErikaHamilton9/status/1797368720076980476
Abdulaziz AlJassim, MD (DrZ_84): “One more Standing Ovation… Durvalumab… A new standard of care” https://twitter.com/DrZ_84/status/1797368826935259189
ADRIATIC Trial design, results, and conclusions
Core design/results (as amplified by KOLs)
Overview amplified by KOLs
- Setting: Limited-stage SCLC after concurrent chemoradiotherapy (cCRT), consolidation durvalumab vs placebo; ± PCI allowed.
- Dual primary endpoints: Overall survival (OS) and progression-free survival (PFS).
- Efficacy: mOS 55.9 vs 33.4 months (HR 0.73); mPFS 16.6 vs 9.2 months (HR 0.76).
- Safety: Grade 3/4 AEs balanced (~24% both arms); pneumonitis noted by several KOLs.
KOL deep-dives on design/results
- Jarushka Naidoo, MD – Emphasizes exact OS/PFS metrics and pneumonitis signals; “first advance in 30+ yrs” framing. https://twitter.com/DrJNaidoo/status/1797369436430594290
#ASCO24 Plenary🔥
— Jarushka Naidoo (@DrJNaidoo) June 2, 2024
Ph III ADRIATIC trial of consolidation Durva +/-Treme post cCRT for LS SCLC:
- benefit in mOS 55.9 v 33.4m (HR 0.73, p=0.01) & mPFS 16.6 v 9.2m
- pneu 10.7% & RT pneu 22.4%*
First advance in 30+yrs in LS SCLC @asco @myESMO @IASLC @OncoAlert @DavidRSpigel #LCSM pic.twitter.com/ocAblSx2Ho
- Shankar Siva, MD, PhD – Calls it “practice changing,” highlights HRs and AE balance from a radiation oncology lens. https://twitter.com/_ShankarSiva/status/1797367226405724564
📢🚨#ASCO24 plenary @DavidRSpigel - pivotal ADRIATIC ph III trial; adjuvant durvalumab arm after chemoradiotherapy (cCRT) ± PCI in small cell #lungcancer; ⬆️ OS (HR 0.73), median OS 55.9 vs 33.4 for placebo. Grade 3/4 AEs in 24.3% vs 24.2%. 💥Practice changing!!! #radonc #lcsm pic.twitter.com/o1BJiUk2C2
— Shankar Siva (@_ShankarSiva) June 2, 2024
- Noemi Reguart, MD – Details dual primary endpoint success and hazard ratios, contextualizing the magnitude of benefit. https://twitter.com/NReguart/status/1797369372844994874
Second practice changing trial of the day: ADRIATIC Durva consolidation vs plb after cCRT in LS-SCLC. Dual P. endpoints OS/PFS meet (3-y FUP): 24 mo OS gain (median 55.9 vs 33.4, HR 0.73) and 24% reduction in the risk of PFS/death (median 16.6 vs 9.2 mo, HR 0.76) #ASCO24 #LCSM pic.twitter.com/YI0yb440FP
— Noemi Reguart (@NReguart) June 2, 2024
Treatment landscape context
- Stephen V. Liu, MD – Notes a “much greater impact on OS … in the limited stage setting than seen in extensive stage,” situating ADRIATIC within prior ES-SCLC IO experience. https://twitter.com/StephenVLiu/status/1797369428226494869
ADRIATIC Trial Sentiments from KOLs: standard of care and critical appraisals
Strongly supportive/SoC-leaning voices
ADRIATIC study is the new standard of care for limited stage small cell lung cancer followed by adjuvant durvalumab with improved mOS to 56 mo from
— Salma Jabbour (@SalmaJabbour1) June 2, 2024
33 mo (placebo) and mPFS of 17 months with durvalumab vs 9 mo (placebo) #ASCO24 pic.twitter.com/ZRqpQMhFLg
Shankar Siva, MD, PhD (practice-changing declaration): https://twitter.com/_ShankarSiva/status/1797367226405724564
Salma Jabbour, MD (“new standard of care” with OS/PFS specifics): https://twitter.com/SalmaJabbour1/status/1797369378771448318
Abdulaziz AlJAssim, MD (DrZ_84) (“A new standard of care”): https://twitter.com/DrZ_84/status/1797368826935259189
SuyogCancer, MD (“This should be standard of care”): https://twitter.com/SuyogCancer/status/1797367479930609744
Erika Hamilton, MD (“transforming results… Big win for patients”): https://twitter.com/ErikaHamilton9/status/1797368720076980476
Other KOL perspectives
Henning Willers, MD (RT strategy/toxicity): “Stunning results‼️ … Make chest RT less toxic or keep trying to dose escalate? … G3+ esophagitis rates remain too high nationally! More or less PCI?” https://twitter.com/HenningWillers/status/1797390500837638186
Corinne Faivre-Finn, MD, PhD (PCI vs MRI surveillance; optimization): “Next key question 👉 PCI+MR surveillance vs MR surveillance … recruit to PRIMALUNG … MAVERICK…” https://twitter.com/finn_corinne/status/1797438492437602317
ADRIATIC Temporal Sentiment Arc
2024 (ASCO 2024 plenary; rapid shift to “practice-changing”) Primary/KOL tweets: https://twitter.com/DrJNaidoo/status/1797369436430594290
https://twitter.com/_ShankarSiva/status/1797367226405724564
https://twitter.com/ASCO/status/1797370147587379373
https://twitter.com/NReguart/status/1797369372844994874
https://twitter.com/n8pennell/status/1797369975868367054
Tone: Immediate enthusiasm with repeated “practice-changing/new SOC” language. KOLs underscore the magnitude of benefit (mOS 55.9 vs 33.4 months, HR 0.73; mPFS 16.6 vs 9.2 months, HR 0.76) and balanced Grade 3/4 AEs. Momentum coalesces quickly toward adoption after cCRT in LS-SCLC.
2024 (ESMO2024)
Tone: Deeper dives and curated explainers spread the findings to broader clinical audiences. Cross specialty discussion of subset analysis from ESMO24 and practical application of the ADRIATIC Trial findings.
ADRIATIC: relative Durva benefit in pre-specified subgroups
— Tom Newsom-Davis (@tnewsomdavis) September 13, 2024
👉PCI: ⬆️ OS overall, but similar Durva benefit
👉Carbo v Cis: Non sig ⬆️ OS benefit
👉BD v OD RT: Comparable OS benefit
🤔 PCI in LS-SCLC better studied in ongoing trials
🤔 Let's stop using cisplatin#ESMO24 #LCSM pic.twitter.com/VpzDILC89j
No! Testing the treatment effect in a subgroup does not carry along with it the covariate adjustment needed, and subgrouping variable BID vs QD probably interacts with an ignored characteristic such as tumor size or nodal extent, and this is grossly misleading! #ESMO24 https://t.co/sMoKYem3Y6
— M. Bolton (@5_utr) September 17, 2024
2025 Discussions: International voices echo sustained adoption and explore center‑specific pathways (RT techniques, PCI vs MRI surveillance, toxicity monitoring). Discussions emphasize patient selection, multidisciplinary coordination, CNS effects, with a key focus on Dr. Suresh Senan's presentation at #ELCC25
In ADRIATIC, durvalumab after cCRT in LE-SCLC delayed time to CNS progression and reduced brain mets — even without PCI. Insightful data shared by Dr. Suresh Senan at #ELCC25. A new horizon for limited-stage SCLC? #SCLC @myESMO pic.twitter.com/Nr2v6ruK0b
— Luis Lara-Mejía (@LuisLara_M) March 29, 2025
Suresh Senan presenting exploratory analysis of #Adriatic study in #lungcancer at #ELCC25. Lowest risk of brainmets in #durva arm, esp when pci #radiotherapy is given as well. pic.twitter.com/V9jlwxWKhK
— Ben Slotman (@bslotman) March 28, 2025
Tone: The arc progresses from pre‑plenary anticipation to a decisive “practice‑changing” consensus at ASCO 2024, followed by an implementation‑focused phase that refines PCI/RT strategies and toxicity surveillance. Through late 2024 and into 2025, international and cross‑disciplinary threads reinforce adoption while iterating on practical optimization and patient selection.
sentiment trend anchor
