Lung Cancer
HARMONi-A
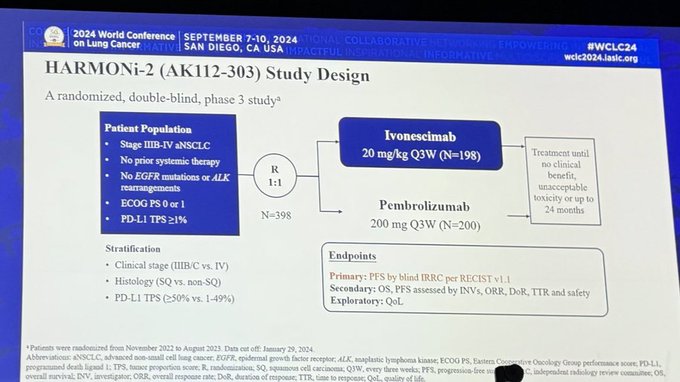
About the HARMONi-A Trial
The HARMONi-A trial is a randomized, double-blind, Phase 3 study comparing ivonescimab to pembrolizumab in patients with stage IIIB–IV advanced non-small cell lung cancer (aNSCLC). Participants were randomized to receive either ivonescimab (20 mg/kg every 3 weeks) or pembrolizumab (200 mg every 3 weeks). The primary endpoint was progression-free survival (PFS) assessed by an independent radiology review committee (IRRC) per RECIST v1.1, while secondary endpoints included overall survival (OS), investigator-assessed PFS, objective response rate (ORR), duration of response (DoR), time to response, and safety. Ivonescimab demonstrated a statistically significant improvement in PFS compared to pembrolizumab, with a hazard ratio of 0.51 and a 5.3-month gain in median PFS. Additionally, ivonescimab showed higher ORR (50.0% vs. 38.5%) and disease control rate (DCR) (89.9% vs. 70.5%) than pembrolizumab. These findings were presented by Prof. Caicun Zhou at the 2024 World Conference on Lung Cancer, held in San Diego, California. Source materials include detailed presentations and subgroup analyses shared during the conference.Table of Contents
Major Presentations and Milestones
HARMONi-A Trial design, results, and conclusions
HARMONi-A Sentiments and Criticisms
HARMONi-A Temporal Sentiment Arc
Professional Resources : Interactive Tweet History, Influence Diagram, Sentiment Table, AI Chatbot
HARMONi-A Trial: Major Presentations and Milestones
Primary speakers driving the story
At ASCO 2024, Prof. Li Zhang presented the phase III HARMONi-A study evaluating ivonescimab (a PD‑1/VEGF bispecific antibody) plus platinum-based chemotherapy versus placebo plus chemotherapy in EGFR‑mutant NSCLC after progression on an EGFR TKI. Oncologists highlighted a statistically significant PFS advantage and debated mechanisms and clinical positioning relative to prior immunotherapy/anti‑VEGF data.
Dr. Li Zhang at #ASCO24 presents phase III HARMONi-A study of chemotherapy + ivonescimab (PD1/VEGF bispecific) vs placebo in pts with #EGFR NSCLC post TKI. https://t.co/0wERcEUHsY
— Stephen V Liu, MD (@StephenVLiu) May 31, 2024
An exclamation point on HARMONi-A results. Looking forward to discussing with folks in the lung cancer (& broader oncology) community at #ASCO24.
— H. Jack West, MD, FASCO (@JackWestMD) May 30, 2024
#LCSM #OncoAlert https://t.co/ebsKrHJzw3
HARMONi-A Trial Design, Results, and Conclusions
Trial Design:
HARMONi-A is a phase III, randomized trial in EGFR‑mutant advanced NSCLC after progression on an EGFR TKI. Patients received ivonescimab (PD‑1/VEGF bispecific) plus platinum doublet chemotherapy versus placebo plus chemotherapy. The primary endpoint was PFS; OS data were immature at initial presentation. The program sits alongside ongoing global “HARMONi” expansion trials.
Primary Results:
ASCO 2024 data showed a significant PFS benefit for ivonescimab plus chemotherapy versus chemotherapy alone: hazard ratio 0.46 (95% CI 0.34–0.62), with benefit described across prespecified subsets; OS was immature at that time. One report summarized an OS signal (with ~52% events) and noted an OS HR reported in session materials.
⏰NOW OUT‼️#ASCO24 Abstracts
— Hidehito HORINOUCHI (@HHorinouchi) May 28, 2024
🔥#8508 HARMONi-A: Chemo +/- Ivonescimab (AK112/SMT112, anti-PD-1/VEGF bispecific ab) in EGFRm NSCLC after EGFR-TKIs resistance
🎙️Dr. Li Zhang
🎯PFS HR 0.46 (95%CI: 0.34, 0.62)
✅Phase III
✅Primary PFS
✅NCT05184712
#LCSM @OncoAlert @ASCO https://t.co/HLh4r4Shgl
HARMONi-A: Large PFS 0.46 benefit and across subgroups including CNS and genotype. Strong OS signal despite immaturity (52% events). TRAEs discontinuation 5.6%. Mostly heme tox. #ASCO24 https://t.co/DvG7N1s1Y5
— Sanjay Popat (@DrSanjayPopat) May 31, 2024
Objective response and disease control favored the ivonescimab arm in session materials: ORR approximately 50.6% versus 35.4% and DCR approximately 93.1% versus 83.2%, with longer median DoR (about 6.6 vs 4.2 months) in the experimental arm (values as shown on shared ASCO slides).
Safety:
Treatment‑related adverse events were common with chemotherapy backbone in both arms; discontinuation of ivonescimab for TRAEs was ~5.6% (versus ~2.5% for placebo). The profile suggested more VEGF‑mediated events (e.g., hypertension) but otherwise similar overall rates; most high‑grade events were hematologic. Session slides also noted grade ≥3 immune‑related events ~6.2% and grade ≥3 VEGF‑related events ~3.1% in the ivonescimab arm.
Key Conclusions:
HARMONi-A demonstrated a clinically meaningful PFS improvement with ivonescimab plus chemotherapy after EGFR‑TKI progression, with consistent signals across subsets at ASCO 2024 and an evolving OS picture. Investigators emphasized careful monitoring for VEGF‑class toxicities and hematologic events, while acknowledging the need for mature OS and CNS‑specific data to refine positioning versus alternative post‑TKI strategies.
HARMONi-A Sentiments and Criticisms
Positive Reception:
Sanjay Popat, MD: "HARMONi-A: Large PFS 0.46 benefit and across subgroups including CNS and genotype. Strong OS signal despite immaturity (52% events). TRAEs discontinuation 5.6%. Mostly heme tox." https://x.com/DrSanjayPopat/status/1796664813327708326
Tom Newsom‑Davis, MD: "Impressive PFS benefit: same as MARIPOSA-2 … None of Amivantamab toxicity issues … Big challenge to Ami" https://x.com/tnewsomdavis/status/1796667348876693651
H. Jack West, MD, FASCO: "An exclamation point on HARMONi-A results. Looking forward to discussing with folks in the lung cancer (& broader oncology) community at #ASCO24." https://x.com/JackWestMD/status/1796250674214355399
Critical Perspectives:
Charu Aggarwal, MD, MPH, FASCO: "@StephenVLiu Important data. I do worry about (lack of) CNS penetration" https://x.com/CharuAggarwalMD/status/1796665615853199794
Tejas Patil, MD contrasted with prior datasets: "I honestly find the HARMONI-A data confusing. ➡️ KEYNOTE-789: no benefit w/ post-osi pembro ➡️ ORIENT-31: sintilimab+bev biosimilar improved PFS post-osi, not OS ➡️ ETOP-BOOSTER: no bev benefit w/ 2L osi But PD1/VEGF bispecific w/ PFS benefit? @EGFRResisters @lcsmchat #asco24" https://x.com/TejasPatilMD/status/1794192418503577628
Nathan A. Pennell, MD, PhD: "I think this is a straight up bev- like effect, agree with @ADesaiMD. Platinum doublet with bev always had modestly longer PFS when randomized against chemo, even possibly OS depending on chemo partner. Not necessarily any PD1 activity needed to see this result." https://x.com/n8pennell/status/1794322674346570084
Stephen V Liu, MD, on bispecific pharmacology: "@TejasPatilMD @EGFRResisters @lcsmchat The binding properties with a bispecific like this are very different though." https://x.com/StephenVLiu/status/1794359662479081525
Jordi Remon, MD emphasized heterogeneity and pending global trials: "For pts w EGFRm with Osi-Progression NSCLC not homogeneous benefit in PFS with🧐antiangiogenic + io+CT vs CT and no mature OS benefit Ph 3 HARMONI-3 trial (ivonescimab (bispecific VEGF/PD1 Ab)+ CT vs CT is pending. Data in Chinese pts looks similar to previous (HARMONI-A) #ASCO24" https://x.com/JordiRemon/status/1796664733694566633
HARMONi-A Temporal Sentiment Arc
2024 (ASCO24 initial efficacy signal)
Primary/KOL tweets:
‼️
— Tom Newsom-Davis (@tnewsomdavis) May 31, 2024
Ivonescimab (PD-1/VEGF) + chemo post Osimertinib
✅ ⬆️ PFS
❓ ⬆️ OS benefit (immature)
✅ ⬆️ ORR
✅ ⬆️ DoR
✅ Minor additional tox vs. chemo
🧐 Impressive PFS benefit: same as MARIPOSA-2
🧐 None of Amivantamab toxicity issues
🧐 Big challenge to Ami 🤜🤜
#ASCO24 #LCSM https://t.co/wB0r6COiZW
- https://x.com/GlopesMd/status/1793763453225545791
- https://x.com/HHorinouchi/status/1795454988254122475
- https://x.com/JackWestMD/status/1796250674214355399
- https://x.com/DrRiyazShah/status/1796664325827928086
- https://x.com/DrSanjayPopat/status/1796664813327708326
- Tone: Strong interest driven by a clear PFS advantage (HR ~0.46) and improved ORR/DoR on ivonescimab + chemotherapy. Early discussion focused on clinical comparators (KEYNOTE‑789, ORIENT‑31, MARIPOSA‑2) and the contribution of VEGF versus PD‑1 mechanisms, alongside calls for mature OS and CNS‑specific data.
- Shift: As slides circulated, attention expanded to safety signals (VEGF‑class AEs, hematologic events) and practical placement after osimertinib, while noting heterogeneous experiences across prior anti‑VEGF/IO datasets.
2025 (OS updates and global context)
Primary/KOL tweets:
Akeso reports Phase III HARMONi-A trial positive for survival. Ivonescimab (PD1/VEGF bispecific) + chemotherapy vs chemo in #EGFR NSCLC post TKI already showed PFS benefit (7.1m vs 4.8m) but OS was in question. Full results to be presented at #WCLC25.
— Stephen V Liu, MD (@StephenVLiu) Aug 28, 2025
https://t.co/6GAlMA2K0N
- https://x.com/JacobPlieth/status/1960650898125750578
- https://x.com/StephenVLiu/status/1960961470138576973
- Tone: Cautious optimism with reports of positive survival, contrasted by skepticism regarding statistical boundaries and generalizability from Chinese cohorts to global programs.
- Shift: From PFS‑led enthusiasm to validation of OS and cross‑trial comparisons informing sequencing against amivantamab‑containing regimens and other anti‑VEGF/IO strategies.
Overall, discourse progressed from first disclosure of a robust PFS signal and improved response metrics to a more nuanced debate about mechanism, toxicity class effects, CNS activity, and the magnitude and robustness of OS benefit as global data mature.
HARMONi-A Professional Resources
