Lung Cancer
Beamion Lung-1
BEAMION Lung-1 Discussion Leaders on X
BEAMION Lung-1 Top Tweets
Presented at #AACR25:
— NEJM (@NEJM) April 28, 2025
Among patients with tumors harboring tyrosine kinase domain mutations who received zongertinib (a selective inhibitor of the HER2 tyrosine kinase), 71% had a response, and median progression-free survival was 12.4 months. Full study results:… pic.twitter.com/QEsXsFxnkP
Zongertinib is now approved for Her2+ NSCLC based off Beamion Lung-1! We had a chance to 🗣️ Her2 testing, trial, findings, AEs and sequencing w/ @JSabari
— Oncology Brothers (@OncBrothers) August 25, 2025
Full 🗣️:
⭐️ https://t.co/Y5WjnOlts8
⭐️ Also on "Oncology Brothers" podcast #OncTwitter #lcsm @OncUpdates pic.twitter.com/FWgOwiwZVc
Developments in HER2 exon 20 ins lung cancer: Boehringer & Bayer see ORRs of 60-70% with zongertinib & BAY2927088, but watch the tox! (Both molecules now in phase 3.) Via @ApexOnco from #WCLC24 -> https://t.co/tyDdvIdCGG pic.twitter.com/b5hW7TeV7f
— Jacob Plieth (@JacobPlieth) September 9, 2024
🎉🎉🎉We celebrate #FDA approval of #zongertinib as the first TKI in #HER2-mutant #NSCLC 🎊 🎂 🌺 🩷
— Xiuning Le MD PhD (@LeXiuning) August 12, 2025
👉 ORR 71%
👉 Duration of Response 14.1 months
👉 PFS 12.4 months
👉 Well-tolerated at 120mg dose
📖 In previously treated patients without prior anti-HER2 targeted therapies… pic.twitter.com/IS6XfykHSI
About the Beamion LUNG-1 Trial
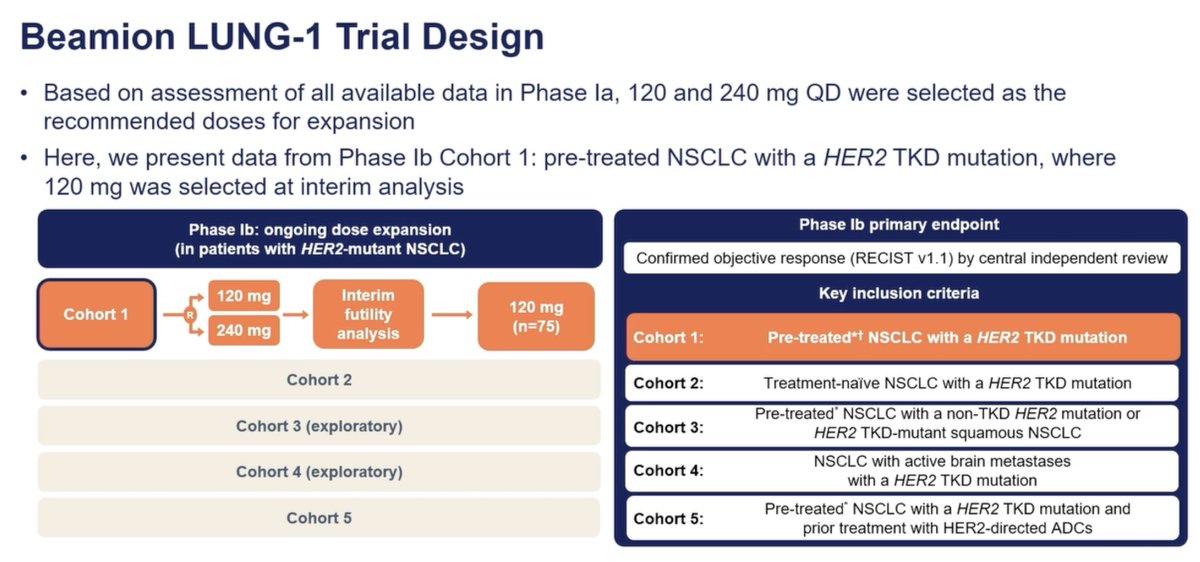
The Beamion LUNG-1 trial is a Phase Ib dose expansion study evaluating the efficacy and safety of a targeted therapy in patients with non-small cell lung cancer (NSCLC) harboring HER2 tyrosine kinase domain (TKD) mutations. Eligible participants were previously treated patients with confirmed HER2 TKD-mutated NSCLC. The primary endpoint was the confirmed objective response rate (ORR) as assessed by central independent review, with secondary endpoints including progression-free survival (PFS) and duration of response (DoR). The trial demonstrated promising results, with ORRs of 72.4% and 78.2% at the 120 mg and 240 mg dose levels, respectively. The safety profile was manageable, with most adverse events being mild to moderate in severity, and diarrhea and rash reported as the most common. Importantly, the investigational therapy also showed efficacy in patients with central nervous system (CNS) metastases. These findings were presented by Dr. Biagio Ricciuti at the 2024 World Conference on Lung Cancer (#WCLC24). Additional insights were shared via social media by Dr. Ricciuti, Dr. Balazs Halmos, and the LUNGevity Foundation.Table of Contents
Major Presentations and Milestones
Beamion Lung-1 Trial design, results, and conclusions
Beamion-Lung-1 Sentiments and Criticisms
Beamion Lung-1 Temporal Sentiment Arc
Major Presentations and Milestones
The next batch of national priority vouchers from @US_FDA
— Anirban Maitra (@Aiims1742) November 6, 2025
Two more oncology products join Daraxonrasib:
•Zongertinib for HER2 lung cancer
•Dostarlimab for rectal cancer
Plus GLP1 agonists…. https://t.co/iowtKDE6hc
#ESMO25 Update on Beamion LUNG-1 study of zongertinib, focused on first-line cohort from @DrSanjayPopat. Zongertinib is a HER2 specific, EGFR sparing TKI FDA approved for previously treated #HER2 mutant NSCLC. Here, cohort 2: TKD mutant, treatment naive. #ESMOAmbassadors pic.twitter.com/8nYEZFNDRA
— Stephen V Liu, MD (@StephenVLiu) October 19, 2025
Primary Speakers Driving the Story
-
Stephen V. Liu, MD (WCLC 2024 live thread; presidential plenary coverage): "Dr. Gerrina Ruiter presents an update on the Beamion LUNG-1 study: zongertinib in #HER2 mutant NSCLC at the #WCLC24 Presidential Plenary. Here, Phase Ib cohort 1: previously treated NSCLC with a #HER2 TKD mutation that set the dose of 120mg by mouth daily." https://x.com/StephenVLiu/status/1833173135539200247
-
Sanjay Popat, MD (WCLC 2024 presenter; early interpretation): "Zongertinib BEAMION-Lung01 pretreated HER2 TKD M+. 120Mg dose chosen. 65% YVMA. 1% g3 diarrhea. Low G3+ TRAEs. ORR 72.4%. Intracranial ORR 33% (RANO). Another highly active HER2 TKI. Looking forward to future development #WCLC24" https://x.com/DrSanjayPopat/status/1833170854756434431
-
Hidehito Horinouchi, MD, PhD (WCLC 2024 presidential plenary highlight): "🔥#WCLC24 Presidential 2 🎙️Dr. Gerrina Ruiter 🎯Primary Phase Ib Analysis of Beamion LUNG-1: Zongertinib (BI 1810631) in Patients with HER2 Mutation-Positive NSCLC #LCSM @IASLC @OncoAlert" https://x.com/HHorinouchi/status/1833170888294068254
-
NEJM (AACR 2025 publication signal): "Presented at #AACR25: Among patients with tumors harboring tyrosine kinase domain mutations who received zongertinib (a selective inhibitor of the HER2 tyrosine kinase), 71% had a response, and median progression-free survival was 12.4 months. Full study results:" https://x.com/NEJM/status/1916841088897409037
KOL Reactions to the Presentation
-
Devika Das, MD: "SOHO1 and BEAMION Lung01… Decent ORR, CNS response +, heavily pretreated pop… Looking forward to subsequent trials. Important to send molecular profile!" https://x.com/DevikaDasMD/status/1833171932281295134
-
Dr. Riyaz Shah: "…Significant GI and skin tox for EGFR sparing; ORR 67%; icORR 33% feels a bit low;" https://x.com/DrRiyazShah/status/1833170519241486718
-
Jacob Plieth (biotech journalist): "…ORRs of 60-70% with zongertinib & BAY2927088, but watch the tox!" https://x.com/JacobPlieth/status/1833168147911774395
-
Kelsey Pan, MD, MPH: "…promising new oral therapies, BAY2927088 and Zongertinib, emerging in the HER2 mutated NSCLC space with intracranial data…" https://x.com/KelseyPanMD/status/1833209118221643803
-
Chul Kim, MD, MPH: "ORR: 72.4% with 120 mg and 78.2% with 240 mg (DoR and PFS immature)… Encouraging intracranial response…" https://x.com/chulkimMD/status/1833170753912803542
Trial Design, Results, and Conclusions Subsection A: Core Design/Results (as amplified by KOLs)
-
Primary endpoints and efficacy framing:
- Stephen V. Liu emphasized Phase Ib cohort 1 in previously treated HER2 TKD-mutant NSCLC, noting the recommended 120 mg daily dose and early efficacy: "RR of 66.7%, after randomization implemented, RR 72.4% at 120mg (vs 78.2% at 240mg). DCR rate 95-100%. DOR and PFS pending (2/3 of pts still on therapy)." Links: https://x.com/StephenVLiu/status/1833173135539200247
#WCLC24 Zongertinib in previously treated #HER2 mutant NSCLC at the recommended 120mg daily oral dose had a RR of 66.7%, after randomization implemented, RR 72.4% at 120mg (vs 78.2% at 240mg). DCR rate 95-100%. DOR and PFS pending (2/3 of pts still on therapy). pic.twitter.com/Hsilunk7Yf
— Stephen V Liu, MD (@StephenVLiu) September 9, 2024
-
- NEJM formalized key outcomes in TKD-mutant tumors: "71% had a response, and median progression-free survival was 12.4 months." Link: https://x.com/NEJM/status/1916841088897409037
-
Safety and tolerability signals (GI/skin, LFTs):
- Stephen V. Liu detailed AE rates at WCLC24: "Diarrhea in 43% but only 1% grade 3 at the 120mg dose. Rash in 24%, ALT elevation in 19% (8% G3), AST 21% (5% G3)." Link: https://x.com/StephenVLiu/status/1833173146096271639
-
Subgroup and molecular context:
- YVMA was the most common TKD mutation and featured high activity (multiple KOLs referenced YVMA):
- "Most common mutation is the YVMA…" (Stephen V. Liu, WCLC24) https://x.com/StephenVLiu/status/1833173146096271639
- "…outstanding RR (up to 90% for key YVMA subset)…" (Balazs Halmos)
2 great presentations on novel ErbB2 TKIs- BAY2927088/zongertinib
— Balazs Halmos (@BalazsHalmosMD) September 9, 2024
☑️outstanding RR (up to 90% for key YVMA subset)
☑️fair tolerance
☑️signal of CNS activity
Main problem we might face…embarrassment of riches- btw tdxd and these 2 exciting agents- which Her-bal therapy to choose? pic.twitter.com/RArcJQewV5
- YVMA was the most common TKD mutation and featured high activity (multiple KOLs referenced YVMA):
KOL deep-dives on design/results with links and framing:
-
Stephen V. Liu, MD (WCLC24 and ESMO25 threads): dose, cohorts, TKD focus, evolving efficacy/AE profile, and CNS signal.
- WCLC24 thread: https://x.com/StephenVLiu/status/1833173135539200247 https://x.com/StephenVLiu/status/1833173146096271639 https://x.com/StephenVLiu/status/1833173164752515364
- ESMO25 first-line thread (cohort 2, treatment-naive TKD): "Included 74 pts… RR 77%, DCR 96% (!), time to response 1.4m… median DOR/PFS not reached, 6m DOR 80%, 6m PFS 79%." https://x.com/StephenVLiu/status/1979819145249304646 https://x.com/StephenVLiu/status/1979819148491596017 https://x.com/StephenVLiu/status/1979819151607955829 https://x.com/StephenVLiu/status/1979819154900500949 https://x.com/StephenVLiu/status/1979819158054568124
-
Oncology Brothers (multi-post breakdowns; regulatory/implementation bridge):
- "Zongertinib (oral TKI) now @US_FDA approved based off #BeamionLung1… 71% ORR… mDoR 14.1m, mPFS 12.4…" (noting ≥Gr3 AEs <20%) https://x.com/OncBrothers/status/1953870964405510192
- "…approved… we had a chance to 🗣️ Her2 testing, trial, findings, AEs and sequencing…" https://x.com/OncBrothers/status/1959987969714168207
- ESMO25 highlights including Beamion LUNG-1: https://x.com/OncBrothers/status/1986460456744329373
Subsets and Deeper Reads
-
Biagio Ricciuti, MD PhD (dose/subgroup safety and CNS details): "120 mg, ORR 72.4%, ≥G3 AEs 17%; 240 mg, ORR 78.2%, ≥G3 AEs 19%… CNS ORR (RANO-BM) 33% and 40%." https://x.com/BiagioRicciutMD/status/1833297767550357986
-
Stephen V. Liu, MD (ESMO25 1L TKD cohort thread with sequential posts; list in order): https://x.com/StephenVLiu/status/1979819145249304646 https://x.com/StephenVLiu/status/1979819148491596017 https://x.com/StephenVLiu/status/1979819151607955829 https://x.com/StephenVLiu/status/1979819154900500949 https://x.com/StephenVLiu/status/1979819158054568124
-
Abdulaziz AlJassim (DrZ_84) (ESMO25 updates across agents; 1L zongertinib): "…77% ORR / 96% DCR in 1L (Beamion LUNG-1) with mostly G1-2 AEs… Phase III Beamion LUNG-2 underway vs SoC" https://x.com/DrZ_84/status/1979475127902642582
Translational/Biomarker Takeaways
- TKD mutation and YVMA subset:
- "Most common mutation is the YVMA…" (Stephen V. Liu) https://x.com/StephenVLiu/status/1833173146096271639
- "…up to 90% for key YVMA subset…" (Balazs Halmos) https://x.com/BalazsHalmosMD/status/1833172404719452483
- CNS efficacy and intracranial activity:
- "…intracranial RR of 41% in Cohort 1… await data from ongoing cohort 4…" https://x.com/StephenVLiu/status/1979819154900500949
- Multiple KOLs citing intracranial response (e.g., Popat: "Intracranial ORR 33% (RANO)"; Chul Kim: "Encouraging intracranial response"). https://x.com/DrSanjayPopat/status/1833170854756434431 https://x.com/chulkimMD/status/1833170753912803542
Subsection D: Practical/Implementation Questions
- Practice positioning and competition (TKIs vs ADC):
- Antonio Calles, MD: "…two new ‘osimertinibs’ for HER2 mutations… hit the 70% response rate threshold… Competitive indication with Trastu-DXd." https://x.com/Tony_Calles/status/1833177671909384440
- Ethical control design and crossover:
- Crispin Hiley, MD, PhD: "Is it ethical to NOT have crossover in the control arm for such highly active drugs targeting HER2 mutations. I think not…" https://x.com/crispinhiley/status/1979202459873505666
- Sequencing and real-world implementation:
- Oncology Brothers (podcast/video on testing, AEs, sequencing): https://x.com/OncBrothers/status/1959987969714168207
- Publication-driven pragmatism:
- Rami Manouchakian, MD ("Hot Off The Press" NEJM thread): https://x.com/RManochakian/status/1916870845030453616
- Dipesh Uprety, MD: "…ORR 71%… mDoR 14.1 mo & mPFS 12.4 mo" https://x.com/DipeshUpretyMD/status/1916868688591946050
Trial Sentiments - Standard of Care and Critical Appraisals
Strongly Supportive/SOC-Leaning Voices
-
Stephen V. Liu, MD (practice-changing tone; threads across WCLC24 and ESMO25): https://x.com/StephenVLiu/status/1979819158054568124
-
Sanjay Popat, MD (high activity, intracranial responses; forward-looking): https://x.com/DrSanjayPopat/status/1833170854756434431
-
Kelsey Pan, MD, MPH (enthusiasm for oral TKIs with CNS data): https://x.com/KelseyPanMD/status/1833209118221643803
-
Oncology Brothers (approval-focused summaries; implementation lens): https://x.com/OncBrothers/status/1953870964405510192
-
NEJM (publication signal consolidating evidence): https://x.com/NEJM/status/1916841088897409037
Critical/Contrarian Perspectives
-
Jacob Plieth: "…ORRs of 60-70%… but watch the tox!" https://x.com/JacobPlieth/status/1833168147911774395
-
Dr. Riyaz Shah: "…Significant GI and skin tox for EGFR sparing… icORR 33% feels a bit low;" https://x.com/DrRiyazShah/status/1833170519241486718
-
Crispin Hiley, MD, PhD: "Is it ethical to NOT have crossover in the control arm for such highly active drugs targeting HER2 mutations. I think not." https://x.com/crispinhiley/status/1979202459873505666
Temporal Sentiment Arc
2024 (WCLC 2024 primary Phase Ib readout; cautious enthusiasm)
PL04.04 - Primary Phase Ib Analysis of Beamion LUNG-1: Zongertinib in HER2+ NSCLC
— Chul Kim (@chulkimMD) September 9, 2024
ORR: 72.4% with 120 mg and 78.2% with 240 mg (DoR and PFS immature)
Encouraging intracranial response noted
Another active drug in this space#WCLC24 pic.twitter.com/6YztITcApH
- Primary URLs: https://x.com/StephenVLiu/status/1833173135539200247 https://x.com/StephenVLiu/status/1833173164752515364 https://x.com/DrSanjayPopat/status/1833170854756434431 https://x.com/chulkimMD/status/1833170753912803542 https://x.com/JacobPlieth/status/1833168147911774395
- Tone summary: Initial WCLC24 reactions highlighted strong response rates at 120/240 mg (ORR 72.4–78.2%) with manageable AEs and encouraging intracranial activity. KOLs debated tolerability (“watch the tox”) and the clinical meaning of icORR ~33–41% while acknowledging promising systemic activity. The field framed zongertinib as an emerging HER2 TKI with a rapidly evolving evidence base.
2025 (AACR 2025 publication; consolidation and precision)
- Primary URLs: https://x.com/NEJM/status/1916841088897409037 https://x.com/RManochakian/status/1916870845030453616 https://x.com/DipeshUpretyMD/status/1916868688591946050 https://x.com/HHorinouchi/status/1915397817897828432
- Tone summary: With NEJM/AACR25, the community aligned around key metrics in TKD-mutant tumors (ORR 71%, median PFS 12.4 months; mDOR ~14.1 months cited by KOLs). Discussion shifted to durability, consistency across subgroups, and safety profiles in the context of clinical adoption. Pragmatic voices began emphasizing trial design rigor and real-world applicability.
2025 (ESMO 2025 first-line cohort; deepening dataset and CNS focus)
HER2-mutant NSCLC updates #ESMO25
— Abdulaziz AlJassim (@DrZ_84) October 18, 2025
•Zongertinib shows 77% ORR / 96% DCR in 1L (Beamion LUNG-1) with mostly G1-2 AEs
•Sevabertinib (SOHO-01 ORR 71%) @LeXiuning
••Phase III Beamion LUNG-2 underway vs SoC 🔥 @DrSanjayPopat pic.twitter.com/8NKx1nRNiE
- Primary URLs: https://x.com/StephenVLiu/status/1979819145249304646 https://x.com/StephenVLiu/status/1979819148491596017 https://x.com/StephenVLiu/status/1979819154900500949 https://x.com/DrZ_84/status/1979475127902642582 https://x.com/crispinhiley/status/1979202459873505666
- Tone summary: First-line cohort data (RR 77%, DCR 96%, 6m DOR 80%, 6m PFS 79%) bolstered enthusiasm and emphasized CNS efficacy signals. Debate intensified around Phase III design ethics (mandatory crossover) and comparative positioning vs SOC and ADCs. The narrative matured toward careful optimism with attention to control design and patient selection.
2025 (Implementation phase: approval, education, and sequencing)
Zongertinib (oral TKI) now @US_FDA approved based off #BeamionLung1 in previously treated metastatic NSCLC w/ HER2+:
— Oncology Brothers (@OncBrothers) August 8, 2025
- 71% ORR w/ 14.1mos mDoR
- mPFS 12.4%
- ≥Gr 3 AEs in less than 20%. Common AEs includied diarrhea and rash#OncTwitter #MedTwitter https://t.co/EjLdQmZ6SQ pic.twitter.com/RXtK5PmE1u
- Primary URLs: https://x.com/OncBrothers/status/1953870964405510192 https://x.com/OncBrothers/status/1959987969714168207 https://x.com/OncBrothers/status/1986460456744329373 https://x.com/Tony_Calles/status/1833177671909384440
- Tone summary: With approval-focused communications, KOLs pivoted to testing pathways, adverse event management, and sequencing relative to ADCs (e.g., T-DXd). Conversations highlighted "embarrassment of riches" in HER2-mutant NSCLC and the need for rational first-line strategies pending Phase III readouts. Practical implementation content (podcasts, videos) reinforced clinician education.
Final Tone: The community evolved from early WCLC24 optimism about response and CNS signals to publication-driven confidence in core outcomes (ORR 70%, median PFS 12 months) and then to nuanced ESMO25 first-line positioning with emphasis on CNS efficacy and trial design ethics. Supportive voices frame zongertinib as potentially practice-changing in HER2 TKD-mutant NSCLC (especially with CNS activity), while critical appraisals continue to focus on toxicity vigilance, intracranial response magnitude, and the necessity of crossover in Phase III to ensure ethical and interpretable results.
Professional resources
BEAMION Lung-1
KOL Sentiment Table
| Doctor Name | Sentiment | Comment |
|---|---|---|
| Dr. Estela Rodriguez | POSITIVE | FDA Grants #Zongertinib Breakthrough Therapy Designation in HER2-Mutant NSCLC ⏭️ Eagerly awaiting for more options for patients w HER2+ NSCLC post T-DXd ⏭️BAY 2927088 also has FDA breakthrough designation #lcsm https://t.co/363GdJihZ4 via @targetedonc |
| Xiuning Le MD PhD | POSITIVE | This is the second BTD for HER2-mut NSCLC, together with BAY88 @Bayer , offering hope to patients! @Exon20Group @EGFRResisters @lungoncdoc FDA Grants Zongertinib Breakthrough Therapy Designation in HER2-Mutant NSCLC https://t.co/EoF6PytsXz via @targetedonc |
| Sanjay Popat | POSITIVE | Zongertinib BEAMION-Lung01 pretreated HER2 TKD M+. 120Mg dose chosen. 65% YVMA. 1% g3 diarrhea. Low G3+ TRAEs. ORR 72.4%. Intracranial ORR 33% (RANO). Another highly active HER2 TKI. Looking forward to future development #WCLC24 https://t.co/BKxA6jKtvX |
| Chul Kim | POSITIVE | PL04.04 - Primary Phase Ib Analysis of Beamion LUNG-1: Zongertinib in HER2+ NSCLC ORR: 72.4% with 120 mg and 78.2% with 240 mg (DoR and PFS immature) Encouraging intracranial response noted Another active drug in this space #WCLC24 https://t.co/6YztITcApH |
| Tom Newsom-Davis | POSITIVE | BEAMION: Zongertinib in HER2 mut ✅ ORR 66.7% ✅ Best DCR = 94 - 100% ❓PFS + DoR immature ✅ Lower Gr3 diarrhoea than BAY2927088 ✅ CNS activity, icRR = 33-40% 💭 More favourable TRAE 💭 ORR similar to SOHO-01 💭 Need PFS data 💭 Ph3 ongoing #WCLC24 #LCSM https://t.co/1ssVNuHBvH |
| Balazs Halmos | POSITIVE | 2 great presentations on novel ErbB2 TKIs- BAY2927088/zongertinib ☑️outstanding RR (up to 90% for key YVMA subset) ☑️fair tolerance ☑️signal of CNS activity Main problem we might face…embarrassment of riches- btw tdxd and these 2 exciting agents- which Her-bal therapy to choose? https://t.co/RArcJQewV5 |
| Devika Das, MD, MSHQS | POSITIVE | SOHO1 and BEAMION Lung01 Both exciting studies with potential options for HER-2 mutation driven NSCLC. ( roughly 2%) Decent ORR, CNS response +, heavily pretreated pop Looking forward to subsequent trials Important to send molecular profile! 🙏🏽#WCLC24 https://t.co/kInihD3bYv |
| Kelsey Pan, MD, MPH | POSITIVE | It's exciting to see promising new oral therapies, BAY2927088 and Zongertinib, emerging in the HER2 mutated NSCLC space with intracranial data for this group at high risk for brain mets. Look forward to seeing further results in later phase studies #WCLC24 https://t.co/OO3ebKOAP6 |
| Dr. Antonio Calles 🫁🚭 | POSITIVE | 💊 It seems like we have two new "osimertinibs" for HER2 mutations. BAY 2927088 and Zongertinib hit the 70% response rate threshold with an acceptable toxicity profile. Already ph3 trials in 1st line HER2mut NSCLC ongoing. Competitive indication with Trastu-DXd. #WCLC24 #LCSM https://t.co/QXUErUWwig |
| Sai-Hong Ignatius Ou | POSITIVE | Zongertinib #esmoasia2024 update. Potentially the first HER2TKI approved for HER2 exon 20 insertions in NSCLC. https://t.co/4rJxV7xyNq |
| Yüksel Ürün | POSITIVE | New era for HER2-mutant lung cancer! Zongertinib delivered a 71% response rate, 12.4 months median PFS, and low-grade toxicity. No ILD reported. Precision hitting its mark! #LungCancer #Oncology #Targeted #cancerresearch @NEJM @AACR @OncoAlert @OncBrothers #AACR25 https://t.co/t0zKyoYLc3 |
| Dr. Antonio Calles 🫁🚭 | POSITIVE | @n8pennell @IASLC 👌 The "osimertinib" for HER2mut NSCLC. |
| Dr. Estela Rodriguez | POSITIVE | @FDAOncology Grants Priority Review to #Zongertinib in #HER2-Mutant NSCLC based on BEAMION-Lung1 trial, ORR 71% Final Decision (PDUFA) not soon enough for pts waiting for these drugs. #lcsm https://t.co/CVNjh3Rxi9 via @targetedonc |
| Dr. NATHAN PENNELL | POSITIVE | Dr. Saltos shares good news about FDA granting priority review of zongertinib for HER2 mutant NSCLC at @IASLC #TTLC25! https://t.co/z0URF4IcoK |
| Balazs Halmos | POSITIVE | Zongertinib- highly effective AND well tolerated ErbB2 TKI WITH CNS activity from Boehringer-Ingelheim- to a thor oncologist this sounds like a beautiful zong …just in German! With RR of >70% and >12m PFS for pretreated pts w ErbB2 TKD-mutated NSCLC, zongertinib delivers a real https://t.co/WNXk6rML7K https://t.co/EHthv3ixX5 |
| Xiuning Le MD PhD | POSITIVE | 🎉🎉🎉We celebrate #FDA approval of #zongertinib as the first TKI in #HER2-mutant #NSCLC 🎊 🎂 🌺 🩷 👉 ORR 71% 👉 Duration of Response 14.1 months 👉 PFS 12.4 months 👉 Well-tolerated at 120mg dose 📖 In previously treated patients without prior anti-HER2 targeted therapies https://t.co/IS6XfykHSI |
| Stephen V Liu, MD | NEUTRAL | #WCLC23 Dr. Noboru Yamamoto discusses Beamion Lung1, phase Ia/Ib of BI 1810631 in #HER2 altered lung cancer. In phase Ia escalation, no MTD, RR in NSCLC 50%. https://t.co/jjNFcZANwi |
| Hidehito HORINOUCHI | NEUTRAL | 🔥#WCLC24 Presidential 2 🎙️Dr. Gerrina Ruiter 🎯Primary Phase Ib Analysis of Beamion LUNG-1: Zongertinib (BI 1810631) in Patients with HER2 Mutation-Positive NSCLC #LCSM @IASLC @OncoAlert https://t.co/Q5L0yj5oBa https://t.co/zjS2IXAfre |
| Stephen V Liu, MD | NEUTRAL | #WCLC24 Zongertinib in previously treated #HER2 mutant NSCLC at the recommended 120mg daily oral dose had a RR of 66.7%, after randomization implemented, RR 72.4% at 120mg (vs 78.2% at 240mg). DCR rate 95-100%. DOR and PFS pending (2/3 of pts still on therapy). https://t.co/Hsilunk7Yf |
| Stephen V Liu, MD | NEUTRAL | Dr. Gerrina Ruiter presents an update on the Beamion LUNG-1 study: zongertinib in #HER2 mutant NSCLC at the #WCLC24 Presidential Plenary. Here, Phase Ib cohort 1: previously treated NSCLC with a #HER2 TKD mutation that set the dose of 120mg by mouth daily. https://t.co/POCIiEiwpI |
| Biagio Ricciuti, MD PhD | NEUTRAL | Primary Phase Ib Analysis of Beamion LUNG-1: Zongertinib (BI 1810631) in Patients with #HER2 Mutation-Positive #NSCLC. @IASLC @OncoAlert 120 mg, ORR 72.4%, ≥G3 AEs 17% 240 mg, ORR 78.2%, ≥G3 AEs 19% 💊AEs leading to discontinuation: 3% 🧠CNS ORR (RANO-BM) 33% and 40% https://t.co/U4qEHWeivR |
| Chul Kim | NEUTRAL | Emering options in HER2 mutant NSCLC: #BAY2927088 and #zongertinib Both represent important advances. Currently being tested in 1st line setting. Understanding & mitigating resistance mechanisms will be important Insightful discussion by Prof. Chee Lee #WCLC24 https://t.co/TACkXmETSl |
| Hidehito HORINOUCHI | NEUTRAL | 🔥#ACLC24 @IASLC Asia🌏 🗣️Oral Abstract Session 1 ✅Chairs: Drs. Duan jianchun, Xiuhao Zhang 🎙️@ThomasW35874311 🎯Zongertinib (BI 1810631) in Patients with HER2-Driven Tumors: Phase Ia and Phase Ib Analysis of Beamion LUNG-1 #LCSM @OncoAlert https://t.co/QiYCsV1ovI https://t.co/M79AFejSdA https://t.co/ffDKYqVpTa |
| Giannis Mountzios | NEUTRAL | No 7: ZONGERTINIB and BAY2927088 HER2mut TKIs Zongertinib and BAY2927088 are both highly selective TKIs of HER2 activating mutations, including the YVMA insertion in exon 20. Both achieved ORR >70% in pretreated pts and are currently being tested in 1L (Beamion-Lung02/SOHO-02)… https://t.co/bUF5EN3ZSK https://t.co/50rPe1ggCx |
| Dipesh Uprety MD FACP | NEUTRAL | Beamion LUNG-1 @NEJM ➡️Pts with advanced or metastatic HER2-mutant NSCLC treated with zongertinib ➡️In a cohort of 75 patients who received zongertinib at a dose of 120 mg: ORR 71% Md DoR 14.1 mo & Md PFS 12.4 mo #LCSM @OncoAlert @BTFCancerNews https://t.co/Upibs5Xa8e |
| Stephen V Liu, MD | NEUTRAL | Zongertinib in #HER2 NSCLC simultaneous publication @NEJM with #AACR25 presentation by Dr. John Heymach. In previously treated HER2 TKD mt NSCLC, RR 71%, duration of response 14.1m, mPFS 12.4m. G3 AEs in only 17% - diarrhea in 56% of pts but only 1 single case of G3 or higher. https://t.co/ZzSoeixFCy |
| Oncology Brothers | NEUTRAL | 3. #BeamionLung1: Ph 1b, Zongertinib (oral TKI, 120/240mg) in previously treated metastatic NSCLC w/ HER2+: - 71% ORR w/ 14.1mis mDoR - mPFS 12.4% - ≥Gr 3 AEs in less than 20%. Common AEs including diarrhea and rash. 4/5 https://t.co/HOwTgiD08o https://t.co/l8dcllDEHe |
| Rami Manochakian MD, FASCO Cancer Education | NEUTRAL | 🔥🚨@OncoAlert Hot Off The Press. Just published @NEJM Results of: ⭐️ #Beamion LUNG-1 phase 1a–1b of: #Zongertinib (#Oral, irreversible, HER2 #TKI) in previously treated #Patients with advanced #HER2-mutant non-small- cell #LungCancer (#NSCLC). 👇🏻 https://t.co/GAgn3eaebG https://t.co/T2Lgcb2TWm |
| Oncology Brothers | NEUTRAL | For now… cross trial comparison is all we have! This will come over and over in our clinics for Her2+ non-small cell lung cancer. Sequencing and AE discussions so that our pts can make informed/shared decision (@jillfeldman4 🙏🏽🙏🏽) ! #Zongertinib #TDxD #lcsm #OncTwitter https://t.co/kG8iHyMyVC https://t.co/zfzgRE4J8D |
KOL |
Pulse Score |
|---|---|
 Oncology Brothers |
29 |
 Dr. Stephen Liu |
23 |
 Dr. Gilberto Lopes |
10 |
 Dr. Bijoy Telivala |
9 |
 Dr. Santhosh Ambika |
9 |
 Dr. Xiuning Le |
8 |
 Dr. Rami Manochakian |
7 |
 Dr. Chul Kim |
7 |
 Dr. Eric Singhi |
6 |
 Dr. Balazs Halmos |
6 |
 Dr. Devika Das |
5 |
 Dr. Estelamari Rodriguez |
5 |
 Dr. Nathan Pennell |
4 |
 Dr. Kyaw Thein |
3 |
 Dr. Fawzi Abu Rous |
3 |
 Dr. Joshua Reuss |
2 |
 Dr. Mark Awad |
2 |
 Dr. Biagio Ricciuti |
2 |
 Dr. Kelsey Pan |
2 |
 Dr. Aakash Desai |
2 |






