Lung Cancer
MARIPOSA
MARIPOSA Trial KOL Discussion Leaders
MARIPOSA Trial Top Tweets
‼️#MARIPOSA study update: Combination therapy shows over ONE-YEAR mOS improvement compared to osimertinib alone.
— Eric K. Singhi, MD (@lungoncdoc) January 7, 2025
Key data to discuss with patients in clinic—but does this simplify first-line treatment decisions? @OncoAlert @OncBrothers @EGFRResisters #lcsm https://t.co/bgjvbmgXAL pic.twitter.com/PlfAer0eBr
MET IHC is a strong predictive factor for the efficacy of amivantamab + lazertinib in pts with EGFRmut NSCLC, independently of the resistance mechanism to osimertinib. PFS 12.2/4.2mo in MET+/-. Might be useful if the 1st line setting becomes crowded (FLAURA2, MARIPOSA…). #ASCO23 pic.twitter.com/MxwovRMdHP
— Benjamin Besse (@BenjaminBesseMD) June 2, 2023
Indeed- as the MARIPOSA regimen will find expanded use with the exciting OS data- side effect management will be key! Get that backpack ready! pic.twitter.com/CvZvP8jgDD
— Balazs Halmos (@BalazsHalmosMD) March 27, 2025
Why would anyone take these toxic shit medicines over the well tolerated Osi for just a PFS benefit. Also if you want PFS just do chemo OSI. It's way cheaper. https://t.co/jeBkhHqQX7
— Vinay Prasad MD MPH (@VPrasadMDMPH) October 23, 2024
About the MARIPOSA Trial
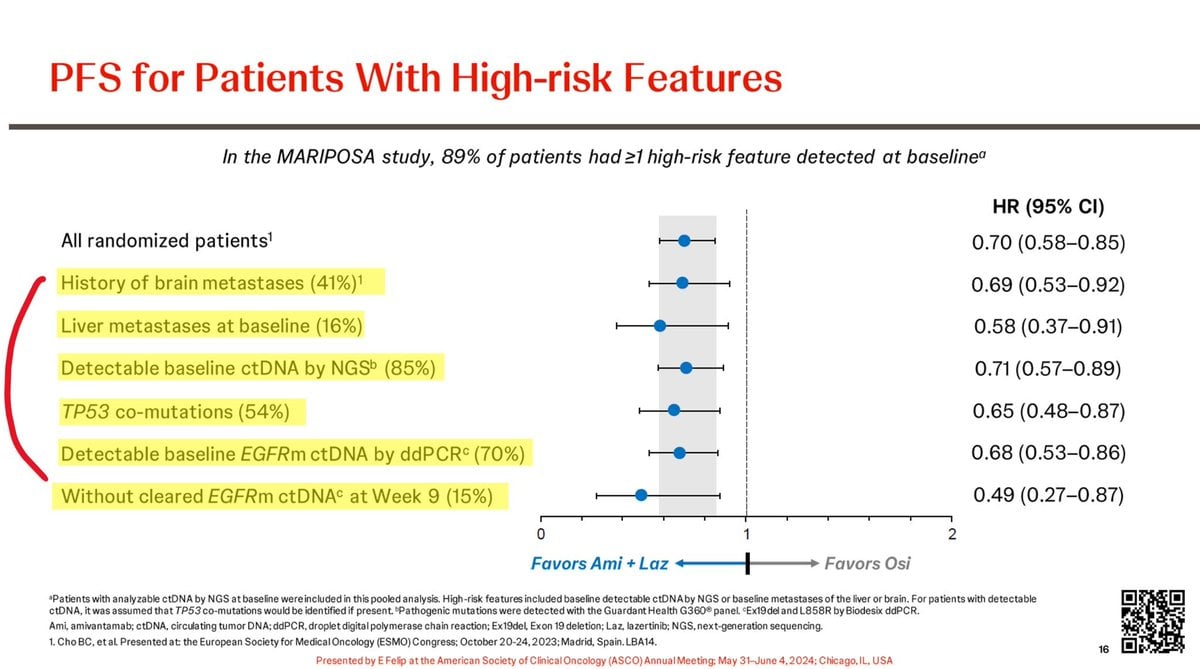
The MARIPOSA trial is a phase 3 clinical study investigating the efficacy of a first-line treatment regimen combining amivantamab, an EGFR-MET bispecific antibody, with lazertinib, a third-generation EGFR tyrosine kinase inhibitor, for patients with advanced EGFR-mutant non-small cell lung cancer (NSCLC). The trial compares this combination to the current standard treatment, osimertinib, focusing on progression-free survival (PFS) and overall survival (OS) outcomes. The study has shown the combination therapy to be effective, offering a statistically significant improvement in median PFS, particularly in high-risk patients with brain metastases, liver metastases, and TP53 co-mutations. These findings suggest a potentially practice-changing treatment option, with ongoing discussions about the safety profiles and management of associated toxicities.
MARIPOSA publication in NEJM
FDA Press Release re: MARIPOSA
Trial Methodology
- Study Design: Phase III, randomized, multi-center trial
- Population: EGFR-mutated advanced NSCLC patients without previous systemic therapy
- Interventions: Comparison between amivantamab plus lazertinib and osimertinib (monotherapy)
- Endpoints: Primary endpoint was progression-free survival (PFS), with secondary endpoints including overall survival (OS), objective response rate (ORR), duration of response (DoR), and safety.

Detailed Results
-
Progression-Free Survival (PFS): The combination of amivantamab and lazertinib demonstrated a significant improvement in PFS compared to osimertinib monotherapy. In patients with high-risk features (such as TP53 co-mutations, liver metastases, and detectable baseline ctDNA), the combination therapy was particularly effective.
-
Overall Survival (OS): The MARIPOSA trial has reported an overall survival benefit of over 12 months.
The MARIPOSA effect 🦋
— Dr. Estela Rodriguez (@Latinamd) March 27, 2025
‼️Awaited OS data for #Amivantamab + lazertinib vs osimertinib in 1L EGFRm advanced #lungcancer presented by Prof. James Yang at #ELCC25
🎯OS HR 0.75 (95%CI: 0.61-0.92)
🎉> 1 yr mOS advantage
🚫no crossover allowed, ⬆️intracranial activity
⬇️toxicity… pic.twitter.com/MZlOq8xCsC -
Safety and Tolerability: While the addition of lazertinib to amivantamab increased toxicity events such as skin disorders and infusion-related reactions, management strategies are being explored to mitigate these effects.
Clinical Implications
The MARIPOSA trial underscores a potential shift in the standard of care, offering an improved therapeutic option for patients with EGFR-mutated NSCLC. The findings emphasize the importance of selecting appropriate patients who may benefit most from combination therapy, particularly those with high-risk disease profiles. Shared decision-making remains crucial in evaluating the benefit-risk profile of this regimen, considering both efficacy and potential adverse effects.
Dynamic debate b/w @JuliaRotow & @BenjaminBesseMD on 1L combos vs 1L osi for #EGFR+ NSCLC
— Sarah Waliany, MD, MS (@SWaliany) July 12, 2025
💠#FLAURA2 & #MARIPOSA more effective for most subgroups than osi alone: highly important for pts w/⬆️risk features
💠Significant tox of MARIPOSA: need for mitigation (subQ ami, COCOON ppx) pic.twitter.com/sbwTUongxp
Key KOL Sentiments for MARIPOSA Trial
KOL PulseRank
MARIPOSA Trial
(Bases on X Activity and Engagement by US Peer Physicians)
KOL |
Pulse Score |
|---|---|
 Dr. Eric Singhi |
135 |
 Dr. Stephen Liu |
116 |
 Dr. Aakash Desai |
69 |
 Oncology Brothers |
62 |
 Dr. Santhosh Ambika |
60 |
 Dr. Gilberto Lopes |
51 |
 Dr. Estelamari Rodriguez |
49 |
 Dr. Balazs Halmos |
41 |
 Dr. Kelsey Pan |
35 |
 Dr. Nathan Pennell |
30 |
 Dr. Rami Manochakian |
29 |
 Dr. Drew Moghanaki |
24 |
 Dr. Chul Kim |
23 |
 Dr. Fawzi Abu Rous |
19 |
 Dr. Bruna Pellini Ferreira |
18 |
 Dr. Vinayak Prasad |
17 |
 Dr. Jarushka Naidoo |
14 |
 Dr. Charu Aggarwal |
14 |
 Dr. Misty Shields |
13 |
 Dr. Bijoy Telivala |
13 |
 Dr. Luis Raez |
13 |
 Dr. Xiuning Le |
12 |
 Dr. Julia Rotow |
12 |
 Dr. Patrick Forde |
11 |
 Dr. Abdul Rafeh Naqash |
10 |
 Dr. Joshua Bauml |
10 |
 Dr. Sarah Waliany |
8 |
 Dr. David Gandara |
8 |
 Dr. Michael Pishvaian |
8 |
 Dr. Chandler Park |
8 |
 Dr. Samuel Kareff |
7 |
 Dr. Sandip Patel |
7 |
 Dr. Thomas Varghese |
7 |
 Dr. Devika Das |
7 |
 Dr. Ana Velazquez Manana |
7 |
 Dr. Edgardo Santos |
5 |
 Dr. Ryan Gentzler |
5 |
 Dr. Patrick Ma |
5 |
 Dr. Narjust Florez |
4 |
 Dr. Gregory Riely |
4 |
 Dr. Tisdrey Torres |
3 |
 Dr. Kyaw Thein |
3 |
 Dr. Tejas Patil |
3 |
 Dr. Zofia Piotrowska |
3 |
 Dr. Ivy Riano Monsalve |
3 |
 Dr. Howard West |
3 |
 Dr. Dipesh Uprety |
3 |
 Dr. Melina Marmarelis |
3 |
 Dr. Coral Olazagasti |
3 |
 Dr. Elad Sharon |
2 |
 Dr. Isabel Preeshagul |
2 |
 Dr. Susan Scott |
2 |
 Dr. Jessica Lin |
2 |
 Dr. Brendan Curley |
2 |
 Dr. David Camidge |
2 |
 Dr. Joe Chang |
2 |
 Dr. Kristen Marrone |
2 |
 Dr. Marina Garassino |
2 |
 Dr. Garrett Green |
2 |
 Dr. Natalie Vokes |
2 |
 Dr. Nicholas Rohs |
1 |
 Dr. Joshua Reuss |
1 |
 Dr. Christian Rolfo |
1 |
 Dr. Bingnan Zhang |
1 |
 Dr. Dawood Findakly |
1 |
 Dr. Sumanta Pal |
1 |
 Dr. Nasser Hanna |
1 |
 Dr. Jasmine Kamboj |
1 |
 Dr. Joshua Sabari |
1 |
 Dr. Nina Maouelainin |
1 |
 Dr. Nagla Karim |
1 |
 Dr. Casey Cosgrove |
1 |
 Dr. Brendon Stiles |
1 |
 Dr. Meilan Han |
1 |
 Dr. Anis Toumeh |
1 |
 Dr. Kaushal Parikh |
1 |
 Dr. Lecia Sequist |
1 |
 Dr. Uzoma Iheagwara |
1 |
 Dr. Jyoti Patel |
1 |
 Dr. David Russler-germain |
1 |
 Dr. Roy Herbst |
1 |
 Dr. Muhammad Zubair Afzal |
1 |
 Dr. Faysal Haroun |
1 |
 Dr. Lyudmila Bazhenova |
1 |
 Dr. Sunil Iyer |
1 |
 Dr. Vamsidhar Velcheti |
1 |
 Dr. Joel Neal |
1 |
 Dr. Sanjay Mukhopadhyay |
1 |
 Dr. Ibrahim Azar |
1 |
 Dr. Vikas Singh |
1 |
 Dr. Amer Zeidan |
1 |
 Dr. Jaskirat Randhawa |
1 |
 Dr. Mara Antonoff |
1 |








