3 min read
CheckMate 8HW YouTube Review: Oncology Brothers with Dr. Alok Khorana
Brian Shields
:
Apr 12, 2025 8:56:27 AM
Insights from the Oncology Brothers Podcast: Dual Checkpoint Inhibition in MSI-High Colorectal Cancer
With the recent FDA approval of the combination of Nivolumab and Ipilimumab for the treatment of MSI-high colorectal cancer, it's important to review the registration trial associated with this important approval.
FDA approves a treatment for patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer. https://t.co/d3NxfgelpK pic.twitter.com/FkId3rT5Mi
— FDA Drug Information (@FDA_Drug_Info) April 8, 2025
YouTube Outline:
| Section | Timestamp | Description |
|---|---|---|
| Study Design | 0:15 | Discusses the Checkmate 8HW study design, including the rationale for testing dual checkpoint inhibition versus single-agent therapy. |
| Study Results | 0:121 | Provides the findings of the study, highlighting the significant improvement in progression-free survival (PFS) in the doublet arms. |
| Adverse Events/Safety | 0:178 | Talks about the increased toxicity associated with dual therapy compared to single agent and the impact on quality of life. |
| Practice Implications | 0:149 | Discusses how the study's findings may change clinical practice and the hope for improved overall survival (OS). |
Study Design
"What Checkmate does is sort of levels up and says, hey, you know, we know one ICI inhibitor is great in the setting. Should we do two? And we know in other settings, you know, dual immune checkpoint inhibitor therapy is better outside of the GI cancer setting." - Dr. Alex Korana. source
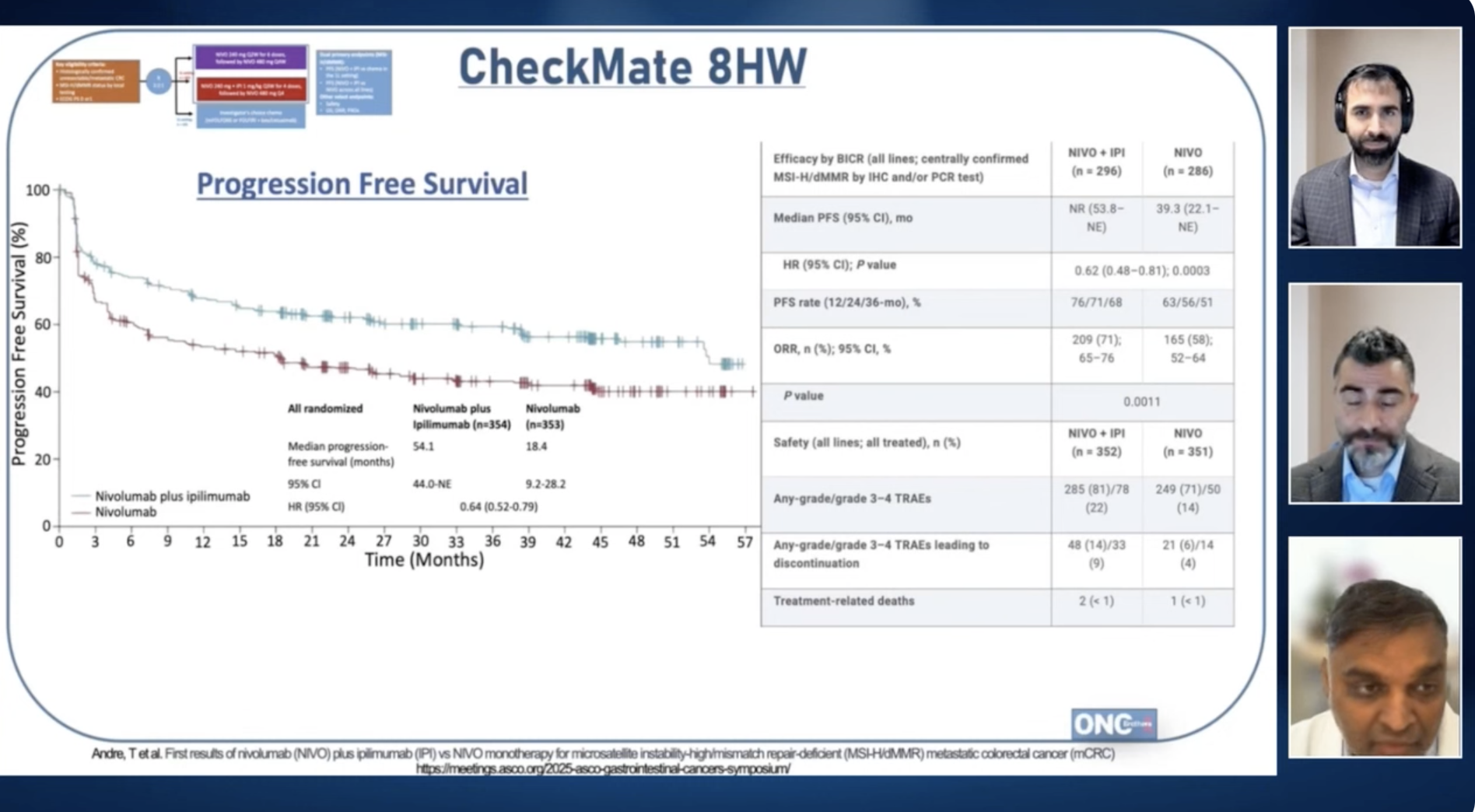
Dr. Khorana detailed the study's design of Checkmate 8HW, which aimed to evaluate the efficacy of dual checkpoint inhibition in MSI-high colorectal cancer patients. As shared by Dr. Khorana, the study explored two therapeutic strategies: the combination therapy of Nivolumab and Ipilimumab versus Nivolumab alone. This design specifically targeted patients with MSI-high, aiming to assess whether dual inhibition could outperform single-agent therapy.

Study Results
"This is just really astounding data, very happy for the patient population that we have improved PFS and hopefully this will lead to improvement in OS as well."-Dr.Alex Khorana source
The conversation revealed impressive findings from the Checkmate 8HW study. Dr. Khorana emphasized the remarkable progression-free survival (PFS) achieved with the dual regimen of Nivolumab and Ipilimumab. Compared to the single-agent strategy of Nivolumab, the combination therapy demonstrated significantly enhanced PFS, underscoring a potentially transformative approach to treating MSI-high colorectal cancer. These results suggest that for this specific subset of patients, dual checkpoint inhibition could herald a shift in treatment paradigms.

Adverse Events/Safety
"I personally have been hesitant to use doublets because of the known increased toxicity associated with doublet immune checkpoint inhibitor therapy and a relatively high response rate and a relatively high duration of response and overall survival. I was reluctant to go down this route " - Dr.Alex Khorana source
In addressing the safety profile, Dr. Alex Khorana and the hosts acknowledged the increased toxicity associated with combined therapy. The podcast underscored the delicate balance between therapeutic efficacy and the management of adverse events. Dr. Rohit Gosain discussed the critical role of shared decision-making in clinical practice, emphasizing the need for clinicians and patients to weigh the potential benefits against the possible impacts on quality of life due to the heightened risk of adverse events with dual therapy.
Shared Decision Making Video Clip
Dr. Rohit Gosain discusses the shared decision making process and the experience that community oncologists have with the Nivolumab and Ipilimumab combination in other cancers
Practice Implications
"...But this Kaplan Meyer curve kind of makes me a believer. I hate to inflict more toxicity on patients, particularly with a heavy burden of disease and that are going to be on treatment for a long period of time." - Dr. Alex Khorana source
The podcast concluded with a discussion on the broader implications of the CheckMate 8HW findings. Dr. Khorana expressed optimism regarding the study's potential to change clinical practice for MSI-high colorectal cancer. With substantially enhanced progression-free survival, clinicians might soon consider adopting dual checkpoint inhibition as a standard treatment, potentially extending overall survival outcomes.
Dr. Khorana also stated that the CheckMate 8DW data were "definitely practice changing" source
What's next? COMMIT Study
Dr. Rohit Gosain reminded viewers of the pending data of the COMMIT study which evaluates if chemotherapy added to immunotherapy may offer benefit to this patient population
Also waiting to see if #COMMIT trial would help address the subset that has early progression with Chemo + Atezo! pic.twitter.com/bGuRuR36hR
— Oncology Brothers (@OncBrothers) April 8, 2025
For those interested in further exploring these insights, please watch to the full discussion in this enlightening episode of the Oncology Brothers podcast.
 Read More
Read More
4 min read
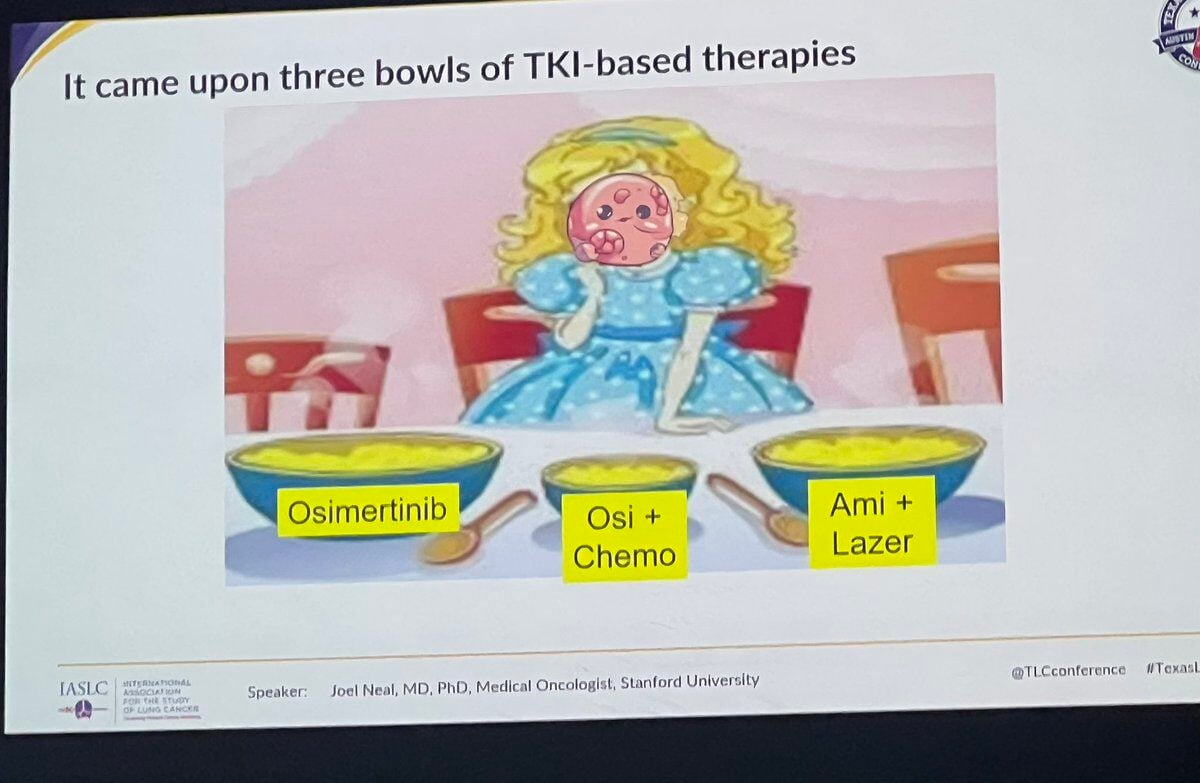
Lung Cancer KOLs Use Memes to Communicate EGFRmut Treatments
The Role of Memes in Communicating Lung Cancer Treatment Challenges: A Focus on EGFR Mutation Therapy In the realm of healthcare communication,...